Division of Molecular Diagnostics
Introduction
Welcome to the Taguchi Lab!
Our laboratory comprises an interdisciplinary team of basic scientists and clinicians who tackle critical issues faced by patients with cancer today.
Our broad interest in solid tumors, particularly pancreatic, colorectal, and lung cancer, involves the identification and validation of diagnostic, predictive, and prognostic blood-based biomarkers, as well as discovery of therapeutic targets. To this end, we apply integrated analyses for the molecular profiling of cancer with particular emphasis on proteomics and utilize primary patient samples, patient-derived xenografts, genetically engineered mouse models, and cancer cell lines. We closely collaborate with our medical colleagues at the Aichi Cancer Center and elsewhere around the world.
The goal of our research is to develop effective diagnostic and therapeutic strategies that will be rapidly translated to have a measurable impact on patients with cancer.
Research topics
We integrate state-of-art molecular profiling technologies to discover biomarkers and therapeutic targets, such as mass spectrometry-based proteomic profiling and NGS, to analyze cancer cell lines, preclinical biospecimens from cancer mouse models, and clinical biospecimens using computational and bioinformatics approaches (Figure 1).
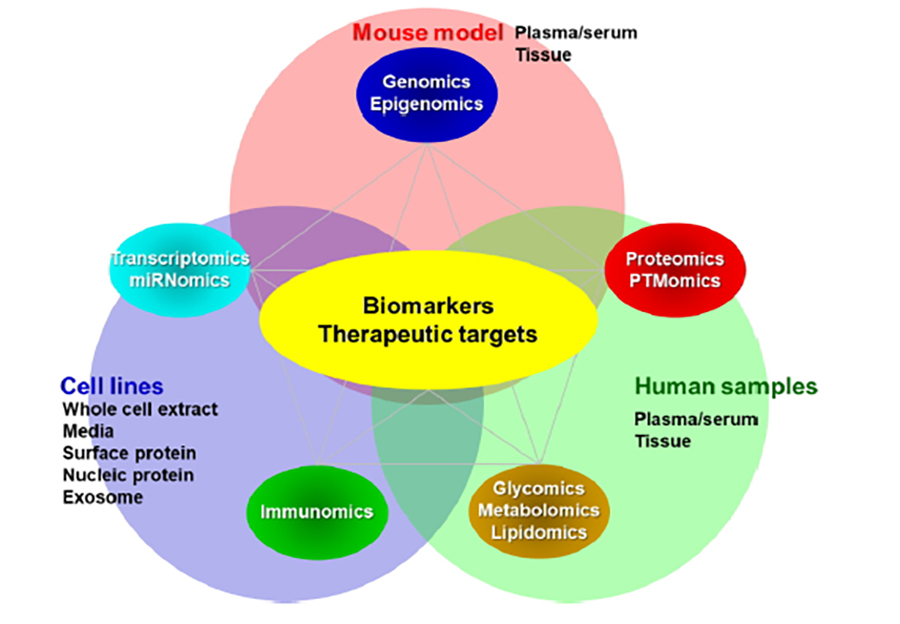
We are currently focusing on the following research projects:
1)Identification and validation of blood-based biomarkers for early detection and prediction of therapy response and prognosis
The rich content of diverse cellular and molecular elements in blood makes it an ideal compartment for developing simple, non-invasive, and cost-effective biomarker tests for cancer, with the potential to assess the risk of developing cancer, identify premalignant lesions or early and potentially curable cancer, distinguish aggressive from indolent tumors, predict and assess therapy responses, and monitor tumor progression and recurrence.
In our previous work, we identified and validated a set of blood-based biomarkers for early detection of lung (Taguchi et al. Cancer Cell 2011; Sin et al. J Clin Oncol 2013; Wikoff et al. J Clin Oncol 2015; Guida et al. JAMA Oncol 2018), colorectal (Taguchi et al. Cancer Prev Res 2015), and pancreatic (Capello et al. JNCI 2017; Fahrman et al. JNCI 2018; Capello et al. Nat Commun 2019) cancers.
We have recently developed an ultra-deep quantitative plasma proteomics approach allowing identification and quantification of more than 2,000 circulating proteins and 1,000 immunoglobulin-bound antigens in a single plasma sample. Discovery studies of blood-based biomarkers using this approach revealed plasma protein signatures that reflect tumor development and tumor biology (Figure 2), prompting us to validate these findings in multiple plasma sample sets and develop integrative biomarker panels with sufficient sensitivity and specificity for intended clinical applications.
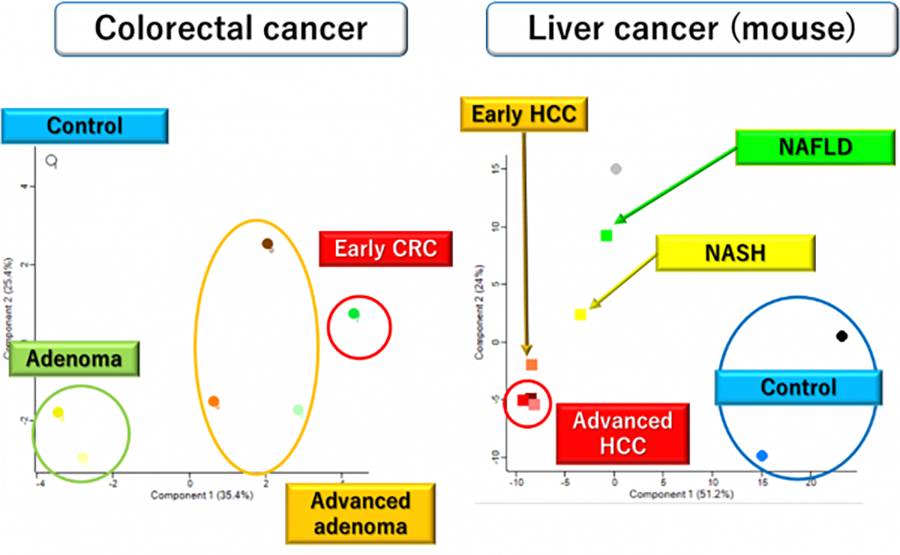
In addition to our continuous efforts to identify early detection biomarkers, we are currently working on identifying blood-based biomarkers for predicting therapy response/recurrence in esophageal, lung, colorectal, and pancreatic cancers.
2)Spatial proteomic profiling for discovery of novel therapeutic targets
To understand molecular mechanisms of cancer, we conducted in-depth quantitative proteomic profiling of cellular compartments in human cancer cell lines with integrated comprehensive genomic and transcriptomic datasets, resulting in identification of molecular features associated with cancer types, mutational status, and various cancer phenotypes such as epithelial vs mesenchymal in lung and pancreatic cancers (Taguchi et al. Cancer Res 2014; Schliekelman et al. Cancer Res 2014; Capello et al. JNCI 2015; Tripathi et al. PNAS 2016; Celiktas et al. JNCI 2017; Tripathi et al. Cancer Res 2018; Yao et al. Nature 2019; Tanaka et al. JNCI 2022).
We have shifted our research focus on cancer cell lines to patient-derived tumor xenograft (PDX) models, as these latter faithfully recapitulate heterogenous human cancer tissues. We have performed multiomics analyses on PDX tumors, including genomics, transcriptomics, and proteomics, with particular emphasis on surfaceome, HLA ligandome, and phosphoproteome (Figure 3), enabling us to further elucidate molecular mechanisms of cancer and identify novel therapeutic targets and biomarker candidates for innovative precision oncology.
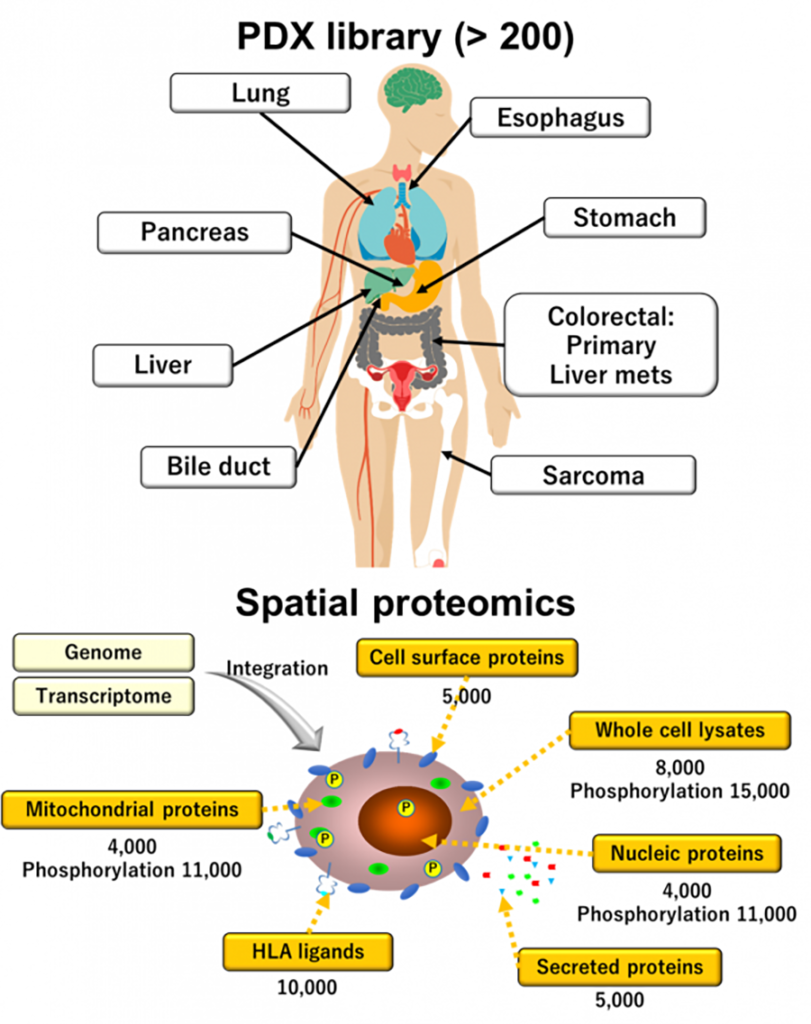
Members
2007-2011 Postdoctoral Fellow, Duke University
2011-2015 Designated Assistant Professor, Graduate School of Medicine, Center for Neural Disease and Cancer Division, Nagoya University
2015-2018 Assistant Professor, Graduate School of Medicine, Center for Research of Laboratory Animals and Medical Research Engineering Division for Advanced Medical Research, Nagoya University
2018-Present. Senior Scientist, Division of Molecular Diagnostics, Aichi Cancer Center
Lab Alumni
- Ichidai Tanaka, 2014–2017 (Postdoctoral Fellow)
- Current position: Assistant Professor, Nagoya University Graduate School of Medicine
- Rekha Jain, 2016–2018 (Postdoctoral Fellow)
- Current position: Assistant Professor, Marwadi University
- Delphine Dayde, 2016–2018 (Postdoctoral Fellow)
- Current position: Postdoctoral Fellow, Dr. Rodolphe Fischmeister’s lab, Inserm
- Mei Chee Tai, 2016–2018 (Postdoctoral Fellow)
- Current position: Postdoctoral Fellow, Cancer Research Malaysia
- Taketo Kato, 2018 (Postdoctoral Fellow)
- Current position: Assistant Professor, Nagoya University Graduate School of Medicine
- Xiaoyi Shi, 2018-2019 (Graduate Student)
- Current position: Ph.D. Graduate Student, Max Planck Institute of Experimental Medicine, Dr. Luis Pardo’s lab
- Behnoush Khaledian, 2018-2019 (Graduate Student)
- Current position: Assistant Professor, Dr. Yohei Shimono’s lab, Fujita Health University
- Yuwen Du, 2018-2021 (Graduate Student)
- Haruki Mori, 2019–2021 (Research Trainee)
- Current position: Assistant Professor, Shiga University of Medical Science
- Kazuyuki Mizuno, 2019–2022 (Research Trainee)
- Current position: Nagoya University Graduate School of Medicine
Publications
2022
- Long non-coding RNA TILR constitutively represses TP53 and apoptosis in lung cancer. Iwai M, Kajino T, Nakatochi M, Yanagisawa K, Hosono Y, Isomura H, Shimada Y, Suzuki M, Taguchi A, Takahashi T. Oncogene. 2022. Online ahead of print.
- A Comprehensive Search of Non-Canonical Proteins in Non-Small Cell Lung Cancer and Their Impact on the Immune Response. Irajizad E, Fahrmann JF, Long JP, Vykoukal J, Kobayashi M, Capello M, Yu CY, Cai Y, Hsiao FC, Patel N, Park S, Peng Q, Dennison JB, Kato T, Tai MC, Taguchi A, Kadara H, Wistuba II, Katayama H, Do KA, Hanash SM, Ostrin EJ. Int J Mol Sci. 23(16):8933. 2022. doi: 10.3390/ijms23168933.
- Mutational Activation of the NRF2 Pathway Upregulates Kynureninase Resulting in Tumor Immunosuppression and Poor Outcome in Lung Adenocarcinoma. Fahrmann JF, Tanaka I, Irajizad E, Mao X, Dennison JB, Murage E, Casabar J, Mayo J, Peng Q, Celiktas M, Vykoukal JV, Park S, Taguchi A, Delgado O, Tripathi SC, Katayama H, Soto LMS, Rodriguez-Canales J, Behrens C, Wistuba I, Hanash S, Ostrin EJ. Cancers. 14(10):2543. 2022. doi: 10.3390/cancers14102543.
- SRGN-Triggered Aggressive and Immunosuppressive Phenotype in a Subset of TTF-1-Negative Lung Adenocarcinomas. Tanaka I, Dayde D, Tai MC, Mori H, Solis L, Tripathi S, Fahrmann J, Unver S, Parhy G, Jain R, Parra E, Murakami Y, Aguilar-Bonavides C, Mino B, Celiktas M, Dhillon D, Casabar J, Nakatochi M, Stingo F, Baladandayuthapani V, Wang H, Katayama H, Dennison J, Lorenzi P, Do KA, Fujimoto J, Behrens C, Ostrin E, Rodriguez-Canales J, Hase T, Fukui T, Kajino T, Kato S, Yatabe Y, Hosoda W, Kawaguchi K, Yokoi K, Chen-Yoshikawa T, Hasegawa Y, Gazdar A, Wistuba I, Hanash S, Taguchi A. J Natl Cancer Inst. 114(2):290-301. 2022. doi: 10.1093/jnci/djab183.
2021
- Identification of Blood-Based Biomarkers for the Prediction of the Response to Neoadjuvant Chemoradiation in Rectal Cancer. Dayde D, Gunther J, Hirayama Y, Weksberg DC, Boutin A, Parhy G, Aguilar-Bonavides C, Wang H, Katayama H, Abe Y, Do KA, Hara K, Kinoshita T, Komori K, Shimizu Y, Tajika M, Niwa Y, Wang YA, DePinho R, Hanash S, Krishnan S, Taguchi A. Cancers. 13(14):3642. 2021. doi: 10.3390/cancers13143642.
- Bioinformatics and Functional Analyses Implicate Potential Roles for EOGT and L-fringe in Pancreatic Cancers. Barua R, Mizuno K, Tashima Y, Ogawa M, Takeuchi H, Taguchi A, Okajima T. Molecules. 26(4):882. 2021. doi: 10.3390/molecules26040882.
- Conditional Ror1 knockout reveals crucial involvement in lung adenocarcinoma development and identifies novel HIF-1α regulator. Isomura H, Taguchi A, Kajino T, Asai N, Nakatochi M, Kato S, Suzuki K, Yanagisawa K, Suzuki M, Fujishita T, Yamaguchi T, Takahashi M, Takahashi T. Cancer Sci. 112(4):1614-1623. 2021. doi: 10.1111/cas.14825.
- Inhibition of heat shock protein 90 destabilizes receptor tyrosine kinase ROR1 in lung adenocarcinoma. Khaledian B, Taguchi A, Shin-Ya K, Kondo-Ida L, Kagaya N, Suzuki M, Kajino T, Yamaguchi T, Shimada Y, Takahashi T. Cancer Sci. 112(3):1225-1234. 2021. doi: 10.1111/cas.14786.
- ST6GAL1 Is a Novel Serum Biomarker for Lenvatinib-Susceptible FGF19-Driven Hepatocellular Carcinoma. Myojin Y, Kodama T, Maesaka K, Motooka D, Sato Y, Tanaka S, Abe Y, Ohkawa K, Mita E, Hayashi Y, Hikita H, Sakamori R, Tatsumi T, Taguchi A, Eguchi H, Takehara T. Clin Cancer Res. 27(4):1150-1161. 2021. doi: 10.1158/1078-0432.CCR-20-3382.
2020
- Novel urinary protein biomarker panel for early diagnosis of gastric cancer. Shimura T, Dayde D, Wang H, Okuda Y, Iwasaki H, Ebi M, Kitagawa M, Yamada T, Yamada T, Hanash SM, Taguchi A, Kataoka H. Br J Cancer. 123(11):1656-1664. 2020. doi: 10.1038/s41416-020-01063-5.
- The Long Noncoding RNA CCAT2 Induces Chromosomal Instability Through BOP1-AURKB Signaling. Chen B, Dragomir MP, Fabris L, Bayraktar R, Knutsen E, Liu X, Tang C, Li Y, Shimura T, Ivkovic TC, Cruz De Los Santos M, Anfossi S, Shimizu M, Shah MY, Ling H, Shen P, Multani AS, Pardini B, Burks JK, Katayama H, Reineke LC, Huo L, Syed M, Song S, Ferracin M, Oki E, Fromm B, Ivan C, Bhuvaneshwar K, Gusev Y, Mimori K, Menter D, Sen S, Matsuyama T, Uetake H, Vasilescu C, Kopetz S, Parker-Thornburg J, Taguchi A, Hanash SM, Girnita L, Slaby O, Goel A, Varani G, Gagea M, Li C, Ajani JA, Calin GA. Gastroenterology. 159(6):2146-2162.e33. 2020. doi: 10.1053/j.gastro.2020.08.018.
- A Promising CPS1 Inhibitor Keeping Ammonia from Fueling Cancer. Taguchi A, Fahrmann JF, Hanash SM. Cell Chem Biol. 27(3):253-254. 2020. doi: 10.1016/j.chembiol.2020.03.002.
- Therapeutic potential of FLANC, a novel primate-specific long non-coding RNA in colorectal cancer. Pichler M, Rodriguez-Aguayo C, Nam SY, Dragomir MP, Bayraktar R, Anfossi S, Knutsen E, Ivan C, Fuentes-Mattei E, Lee SK, Ling H, Catela Ivkovic T, Huang G, Huang L, Okugawa Y, Katayama H, Taguchi A, Bayraktar E, Bhattacharya R, Amero P, He WR, Tran AM, Vychytilova-Faltejskova P, Klec C, Bonilla DL, Zhang X, Kapitanovic S, Loncar B, Gafa R, Wang Z, Cristini V, Hanash SM, Bar-Eli M, Lanza G, Slaby O, Goel A, Rigoutsos I, Lopez-Berestein G, Calin GA. Gut. 69(10):1818-1831. 2020. doi: 10.1136/gutjnl-2019-318903.
2019
- Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Yao W, Rose JL, Wang W, Seth S, Jiang H, Taguchi A, Liu J, Yan L, Kapoor A, Hou P, Chen Z, Wang Q, Nezi L, Xu Z, Yao J, Hu B, Pettazzoni PF, Ho IL, Feng N, Ramamoorthy V, Jiang S, Deng P, Ma GJ, Den P, Tan Z, Zhang SX, Wang H, Wang YA, Deem AK, Fleming JB, Carugo A, Heffernan TP, Maitra A, Viale A, Ying H, Hanash S, DePinho RA, Draetta GF. Nature. 568(7752):410-414. 2019. doi: 10.1038/s41586-019-1062-1.
- Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Capello M, Vykoukal JV, Katayama H, Bantis LE, Wang H, Kundnani DL, Aguilar-Bonavides C, Aguilar M, Tripathi SC, Dhillon DS, Momin AA, Peters H, Katz MH, Alvarez H, Bernard V, Ferri-Borgogno S, Brand R, Adler DG, Firpo MA, Mulvihill SJ, Molldrem JJ, Feng Z, Taguchi A, Maitra A, Hanash SM. Nat Commun. 10(1):254. 2019. doi: 10.1038/s41467-018-08109-6.
- Harnessing Immune Response to Malignant Lung Nodules. Promise and Challenges. Taguchi A, Arenberg D. Am J Respir Crit Care Med. 199(10):1184-1186. doi: 10.1164/rccm.201811-2188ED.
- A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. Fahrmann JF, Bantis LE, Capello M, Scelo G, Dennison JB, Patel N, Murage E, Vykoukal J, Kundnani DL, Foretova L, Fabianova E, Holcatova I, Janout V, Feng Z, Yip-Schneider M, Zhang J, Brand R, Taguchi A, Maitra A, Brennan P, Max Schmidt C, Hanash S. J Natl Cancer Inst. 111(4):372-379. 2019. doi: 10.1093/jnci/djy126.
2018
- Assessment of Lung Cancer Risk on the Basis of a Biomarker Panel of Circulating Proteins. Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Consortium for Early Detection of Lung Cancer, Guida F, Sun N, Bantis LE, Muller DC, Li P, Taguchi A, Dhillon D, Kundnani DL, Patel NJ, Yan Q, Byrnes G, Moons KGM, Tjonneland A, Panico S, Agnoli C, Vineis P, Palli D, Bueno-de-Mesquita B, Peeters PH, Agudo A, Huerta JM, Dorronsoro M, Barranco MR, Ardanaz E, Travis RC, Byrne KS, Boeing H, Steffen A, Kaaks R, Husing A, Trichopoulou A, Lagiou P, La Vecchia C, Severi G, Boutron-Ruault MC, Sandanger TM, Weiderpass E, Nost TH, Tsilidis K, Riboli E, Grankvist K, Johansson M, Goodman GE, Feng Z, Brennan P, Johansson M, Hanash SM. JAMA Oncol. 4(10):e182078. 2018. doi: 10.1001/jamaoncol.2018.2078.
- HIV Infection and Circulating Levels of Prosurfactant Protein B and Surfactant Protein D. Shiels MS, Kirk GD, Drummond MB, Dhillon D, Hanash SM, Taguchi A, Engels EA. J Infect Dis. 217(3):413-417. 2018. Doi: 10.1093/infdis/jix510.
Selected publications until 2017
- Role of CPS1 in Cell Growth, Metabolism and Prognosis in LKB1-Inactivated Lung Adenocarcinoma. Celiktas M, Tanaka I, Tripathi SC, Fahrmann JF, Aguilar-Bonavides C, Villalobos P, Delgado O, Dhillon D, Dennison JB, Ostrin EJ, Wang H, Behrens C, Do KA, Gazdar AF, Hanash SM, Taguchi A. J Natl Cancer Inst. 109(3):1-9. 2017. doi: 10.1093/jnci/djw231.
- Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. Capello M, Bantis LE, Scelo G, Zhao Y, Li P, Dhillon DS, Patel NJ, Kundnani DL, Wang H, Abbruzzese JL, Maitra A, Tempero MA, Brand R, Firpo MA, Mulvihill SJ, Katz MH, Brennan P, Feng Z, Taguchi A, Hanash SM. J Natl Cancer Inst. 109(4):djw266. 2017. doi: 10.1093/jnci/djw266.
- Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Tripathi SC, Peters HL, Taguchi A, Katayama H, Wang H, Momin A, Jolly MK, Celiktas M, Rodriguez-Canales J, Liu H, Behrens C, Wistuba II, Ben-Jacob E, Levine H, Molldrem JJ, Hanash SM, Ostrin EJ. Proc Natl Acad Sci U S A. 113(11):E1555-64. 2016. doi: 10.1073/pnas.1521812113.
- Diacetylspermine Is a Novel Prediagnostic Serum Biomarker for Non-Small-Cell Lung Cancer and Has Additive Performance With Pro-Surfactant Protein B. Wikoff WR, Hanash S, DeFelice B, Miyamoto S, Barnett M, Zhao Y, Goodman G, Feng Z, Gandara D, Fiehn O, Taguchi A. J Clin Oncol. 33(33):3880-6. 2015. doi: 10.1200/JCO.2015.61.7779.
- Carboxylesterase 2 as a Determinant of Response to Irinotecan and Neoadjuvant FOLFIRINOX Therapy in Pancreatic Ductal Adenocarcinoma. Capello M, Lee M, Wang H, Babel I, Katz MH, Fleming JB, Maitra A, Wang H, Tian W, Taguchi A, Hanash SM. J Natl Cancer Inst. 107(8):djv132. 2015. doi: 10.1093/jnci/djv132.
- Pro-surfactant protein B as a biomarker for lung cancer prediction. Sin DD, Tammemagi CM, Lam S, Barnett MJ, Duan X, Tam A, Auman H, Feng Z, Goodman GE, Hanash S, Taguchi A. J Clin Oncol. 31(36):4536-43. 2013. doi: 10.1200/JCO.2013.50.6105.
- Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Taguchi A, Politi K, Pitteri SJ, Lockwood WW, Faca VM, Kelly-Spratt K, Wong CH, Zhang Q, Chin A, Park KS, Goodman G, Gazdar AF, Sage J, Dinulescu DM, Kucherlapati R, Depinho RA, Kemp CJ, Varmus HE, Hanash SM. Cancer Cell. 20(3):289-99. 2011. doi: 10.1016/j.ccr.2011.08.007.
- The grand challenge to decipher the cancer proteome. Hanash S, Taguchi A. Nat Rev Cancer. 10(9):652-60. 2010. doi: 10.1038/nrc2918.
Education & Training
A tutorial in the Taguchi lab provides a unique opportunity for wide exposure to cutting-edge systems-based genomic and proteomic methodologies and experience in basic and advanced molecular and cellular biological techniques, including tissue culture, gel electrophoresis and western blotting, immunoprecipitation, nucleic acid isolation, RT-PCR, mammalian vector construction and transfection, in vitro cell-based assays, fluorescence microscopy, and mouse xenografting.
We are looking for highly motivated graduate students, postdocs, and clinical fellows who want to address fundamental questions in cancer biology and bridge the gap between basic science and clinical practice. The Taguchi lab is located on the fourth floor of the Main building of ACCRI, and provides a rich academic atmosphere and collaborative and fostering environment for our lab’s research.
Postdoctoral fellows/Scientist
Candidates should be self-motivated, ethical, and team-spirited, as well as hold a recent Ph.D. and/or M.D. Previous experience in mouse modeling, mass spectrometry, cell culture, and/or immunoassays are highly desirable. Please contact Dr. Ayumu Taguchi by email (a.taguchi@aichi-cc.jp) and include a cover letter describing your research interests, a CV, and contact information for 2–3 references.
Graduate students
The lab accepts students through the Global 30 International Program at Nagoya University (http://admissions.g30.nagoya-u.ac.jp/graduate/).

