Division of Translational Oncoimmunology
Introduction
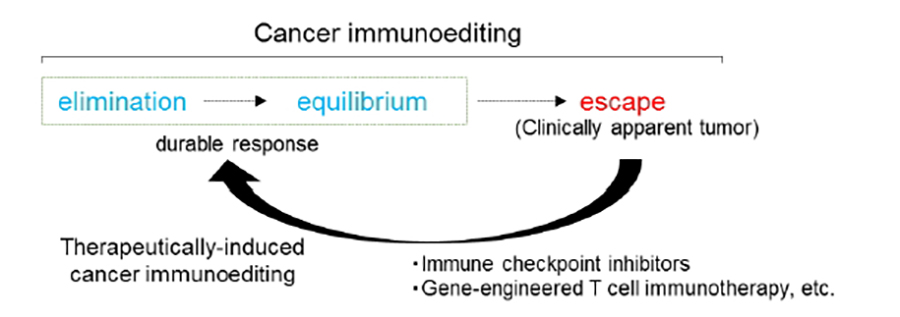
Whether the immune system can recognize and eliminate nascent transformed cells that develop in the body, i.e., cancer immunosurveillance, has been debated for most of the 20th century. In 2001, Robert Schreiber revealed the existence of immunosurveillance in mouse (Shankaran V et al, Nature, 2001) and developed the concept of “cancer immunoediting” (Dunn GP et al, Nat Immunol, 2002). The process of cancer immunoediting consists of three phases: elimination, equilibrium, and escape. Accumulating data from basic research and latest comprehensive multi-omics analysis have demonstrated that immunoediting occurs not only in mice but also in humans.
Cancer immunotherapy was selected as the Breakthrough of the Year by Science magazine in 2013 for the development of immune checkpoint inhibitors (ICIs) and gene-engineered T cell immunotherapy. Dr. James P. Allison and Dr. Tasuku Honjo received the Nobel Prize in 2018 for the discovery and clinical application of ICIs. Cancer immunotherapy has attracted increased attention for cancer treatment, but ICIs remain effective only in a minority of patients. However, the efficacy of ICIs, which is characterized by durable responses that have never been seen before, has shown that host immunity, if re-activated, can eliminate cancer cells or maintain cancer in an equilibrium state (therapeutically induced cancer immune elimination or equilibrium).
Our laboratory is focused on understanding the molecular and cellular basis of natural and therapeutically induced cancer immunoediting in human. We will develop effective cancer immunotherapies combined with ICIs by collaborating with the Aichi Cancer Center Hospital.
Research topics
Understanding T cells and antigenic targets in cancer immunoediting
To improve the therapeutic efficacy of cancers, it is necessary to determine the mechanisms of naturally occurring and therapeutically induced cancer immune elimination or equilibrium in detail and develop immunotherapeutic strategies based on these mechanisms (Fig.1).
T cells are critical effecter immune cells in cancer elimination, and mutation-derived neoantigens are critical tumor antigens in natural responses to cancer in mice (Matsushita H et al, Nature, 2012). However, the underlying mechanisms are poorly understood, particularly in the human clinical setting, such as how many tumor antigens are related to cancer elimination, whether immunodominance of antigens exist in humans, and whether T cells recognizing neoantigens can control cancer for a long time. To address this, we utilize clinical samples including tumor tissues, lymph nodes, and peripheral blood, as well as clinical information from patients who underwent surgery/biopsy. We are focusing on adaptive CD4/CD8 T cells and tumor-specific neoantigens recognized by T cells. We also investigate immune responses induced by therapies such as ICIs to evaluate secondary cancer immunoediting.
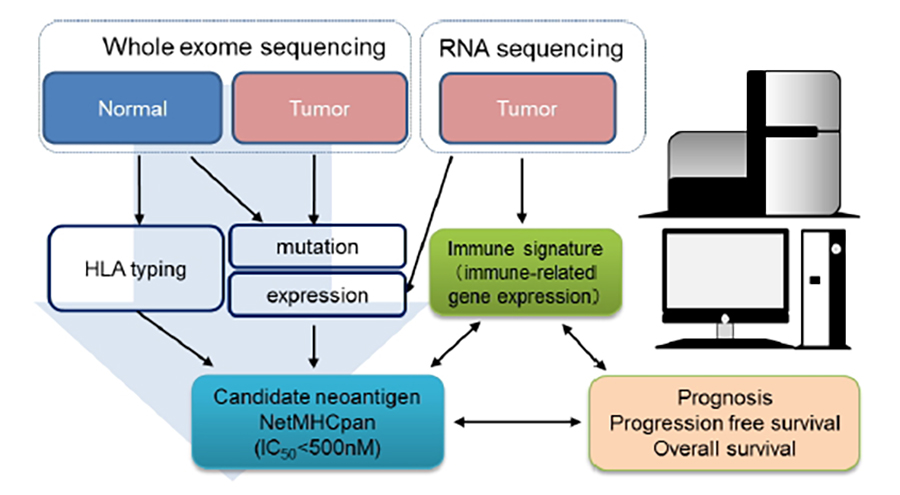
Challenges to neoantigen identification using immunogenomic approaches
To identify the individual somatic mutations in whole exome/RNA sequencing data and predict candidate neoantigens derived from somatic mutations using in silico algorithm (Fig.2).
Immune responses against candidate MHC class I and II epitope peptides were confirmed by conventional cytokine assays, multimer staining, or TCR repertoire analysis using tumor-infiltrating T cells or peripheral blood mononuclear cells (PBMCs) from patients with cancer.
Particularly, we focus on the quality of neoantigens related to long-term survival. We will also determine whether long-lived memory CD4 and CD8 T cells against neoantigens exist in the peripheral blood in long-term responder patients upon treatment with ICIs.
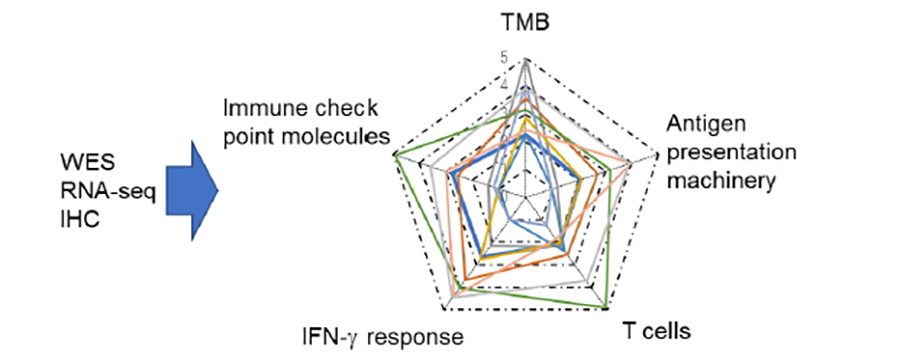
Patient selection and biomarkers for cancer immunotherapy
To select patients who may benefit from cancer immunotherapy, we are comprehensively examining immune responses to cancer by evaluating immune-related gene expression and conducting immunohistochemistry analyses. We evaluate the “cancer immunogram” based on the understanding of cancer-immune interactions to determine the selection criteria for cancer vaccines (Fig.3).
We will evaluate and compare the immune statuses of long-termed responder, short-term responder, and non-responder cases treated with ICIs to understand the immune resistance mechanisms such as antigen loss, antigen presentation machinery (APM) defect, IFN insensitivity, expression of immune checkpoint molecules, oncogene-driven immune suppression, etc.
Development of cancer immunotherapy targeting neoantigens
In 2017, the results of two independent clinical trials of peptide or RNA vaccine therapies targeting neoantigens were reported in malignant melanoma (Ott PA et al, Nature, 2017; Sahin U et al, Nature, 2017). In the future, additional clinical trials of cancer vaccines targeting neoantigens are expected. Future challenges include improving neoantigen identification methods and developing a sensitive assay system for detecting neoantigens as well as determining patient selection criteria for the cancer vaccine. We will work to solve these problems by collaborating with the Aichi Cancer Center Hospital.
Members
2003-2005: Kobe University (2005 M.S.)
2013-2016: Mie University Graduate School of Medicine (2016 Ph.D.)
2006-2013: Researcher, Immunofrontier, Inc.
2013-2015: Assistant Professor, Mie University Graduate School of Medicine
2016-2018: Research Assistant Professor, Graduate School of Pharmaceutical Sciences, University of Shizuoka
2018-2021: Associate Professor, Nagasaki University Graduate School of Biomedical Sciences
2022-present: Unit Leader, Division of Translational Oncoimmunology, Aichi Cancer Center Research Institute
2) Development of novel therapeutic strategies for cancers resistant to immunotherapy.
2) Improving TCR affinity specific for neoantigens
2003-2004 Residency, General Surgery, Keio University Hospital
2005-2008 Fellow, General Thoracic Surgery, Keio University Hospital
2010 Ph.D. from Keio University Graduate School of Medicine
2010 National Cancer Center Hospital East
2011 Assistant Professor, Keio University School of Medicine (General Thoracic Surgery)
2012 Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital
2013-2015 Assistant Professor, Teikyo University School of Medicine (General Thoracic Surgery)
2015-2017 Visiting Researcher, Memorial Sloan Kettering Cancer Center (Thoracic Surgery)
2017 Assistant Professor, Keio University School of Medicine (General Thoracic Surgery)
2018 Chief, Sagamihara Kyodo Hospital (Thoracic Surgery)
2019- Senior Researcher, Division of Translational Oncoimmunology, Aichi Cancer Center Research Institute
2) Biomarkers for immunotherapy responses
3) Immune responses in lung cancer
2008-2010 Residency, Seirei Hamamatsu General Hospital
2010-2015 Department of Obstetrics & Gynecology, Seirei Hamamatsu General Hospital
2015-2022 Department of Gynecologic Oncology, Saitama Medical University International Medical Center
2022- Present Researcher, Aichi Cancer Center Research Institute
2) Clarifying the mechanisms of cancer immunoediting in humans
2013-2015 Residency, Disaster Medical Center, Tokyo, Japan
2015-2016 Clinical training, General Surgery, JA Toride Medical Center, Ibaraki, Japan
2016 Fellow, General Thoracic Surgery, Tokyo Medical and Dental University, Medical Hospital, Tokyo, Japan
2017-2018 Residency, General Thoracic Surgery, Aichi Cancer Center Hospital, Aichi, Japan
2019 Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital, Tokyo, Japan
2020 Fellow, General Thoracic Surgery, Tokyo Medical and Dental University, Medical Hospital, Tokyo, Japan
2021- Present Research Resident, Aichi Cancer Center Research Institute
2) Clarifying the mechanisms of cancer immunoediting in humans
2017-2019 Residency, Tsuyama Chuo Hospital
2019-2021 Residency, Neurological Surgery, Tsuyama Chuo Hospital
2021-2022 Residency, Neurological Surgery, Okayama University Hospital
2022- Research Resident, Division of Translational Oncoimmunology, Aichi Cancer Center Research Institute
2) Elucidating the glioma tumor microenvironment
Publications
- Masago K, Kuroda H, Takahashi Y, Oya Y, Sasaki E, Sakakura N, Matsushita H. Synchronous driver gene alterations (EGFR L858R, T790M, and ROS1) rearrangements in a patient with early-stage lung adenocarcinoma. Cancer Genet. 268-269:124-127. 2022
- Masago K, Kuroda H, Sasaki E, Fujita S, Shinohara S, Sugita Y, Takahashi Y, Matsushita H. Association of the KRAS genotype and clinicopathologic findings of resected non-small-cell lung cancer: A pooled analysis of 179 patients. Cancer Genet. 268-269:64-74. 2022
- Masago K, Kuroda H, Fujita S, Sasaki E, Takahashi Y, Shinohara S, Matsushita H. Biological difference between L858R and exon 19 deletion contributes to recurrence-free survival of resected non-small cell lung cancer. Oncology. 2022 Sep 13.
- Muraoka D, Harada N, Shiku H, Akiyoshi K. Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance. J Control Release. 347:175-182. 2022
- Dotsu Y, Muraoka D, Ogo N, Sonoda Y, Yasui K, Yamaguchi H, Yagita H, Mukae H, Asai A, Ikeda H. Chemical augmentation of mitochondrial electron transport chains tunes T cell activation threshold in tumors. J Immunother Cancer. 10(2):e003958. 2022
- Sato S, Matsushita H, Shintani D, Kobayashi Y, Fujieda N, Yabuno A, Nishikawa T, Fujiwara K, Kakimi K, Hasegawa K. Association between effector-type regulatory T cells and immune checkpoint expression on CD8+ T cells in malignant ascites from epithelial ovarian cancer. BMC Cancer. 22(1):437. 2022
- Shinohara S, Takahashi Y, Komuro H, Matsui T, Sugita Y, Demachi-Okamura A, Muraoka D, Takahara H, Nakada T, Sakakura N, Masago K, Miyai M, Nishida R, Shomura S, Shigematsu Y, Hatooka S, Sasano H, Watanabe F, Adachi K, Fujinaga K, Kaneda S, Takao M, Ohtsuka T, Yamaguchi R, Kuroda H, Matsushita H. New evaluation of the tumor immune microenvironment of non-small cell lung cancer and its association with prognosis. J Immunother Cancer. 10(4):e003765. 2022
- Masago K, Fujita S, Oya Y, Takahashi Y, Matsushita H, Sasaki E, Kuroda H. Comparison between Fluorimetry (Qubit) and Spectrophotometry (NanoDrop) in the Quantification of DNA and RNA Extracted from Frozen and FFPE Tissues from Lung Cancer Patients: A Real-World Use of Genomic Tests. Medicina (Kaunas). 57(12):1375. 2021
- Kuroda H, Ichinose J, Masago K, Takahashi Y, Nakada T, Nakao M, Okumura S, Hashimoto K, Matsuura Y, Sakakura N, Matsushita H, Mun M. Permissible Outcomes of Lobe-Specific Lymph Node Dissection for Elevated Carcinoembryonic Antigen in Non-Small Cell Lung Cancer. Medicina (Kaunas). 57(12):1365. 2021
- Yoshikawa T, Wu Z, Inoue S, Kasuya H, Matsushita H, Takahashi Y, Kuroda H, Hosoda W, Suzuki S, Kagoya Y. Genetic ablation of PRDM1 in antitumor T cells enhances therapeutic efficacy of adoptive immunotherapy. Blood 139(14):2156-2172. 2022
- Kuroda H, Masago K, Takahashi Y, Fujita S, Sasaki E, Nakada T, Sakakura N, Nakanishi H, Matsushita H, Yatabe Y. Positive Correlation Between the Number of Circulating Tumor Cells in the Pulmonary Vein and Tumor Spread Through Air Spaces in Resected Non-small Cell Lung Cancer. Anticancer Res. 41(11):5499-5505. 2021
- Kuroda H, Takahashi Y, Shirai S, Takahara H, Nakada T, Sakakura N, Matsushita H. Survival benefit of immune checkpoint inhibitor monotherapy in patients with non-small cell lung cancer recurrence after completely pulmonary resection. Ann Transl Med. 9(15):1225. 2021
- Nishida M, Yamashita N, Ogawa T, Koseki K, Warabi E, Ohue T, Komatsu M, Matsushita H, Kakimi K, Kawakami E, Shiroguchi K, Udono H. Mitochondrial reactive oxygen species trigger metformin-dependent antitumor immunity via activation of Nrf2/mTORC1/p62 axis in tumor-infiltrating CD8T lymphocytes. J Immunother Cancer. 9(9):e002954. 2021
- Kuroda H, Sugita Y, Masago K, Takahashi Y, Nakada T, Sasaki E, Sakakura N, Yamaguchi R, Matsushita H, Hida T. Clinical Guideline-Guided Outcome Consistency for Surgically Resected Stage III Non-Small Cell Lung Cancer: A Retrospective Study. Cancers (Basel).13(11):2531. 2021
- Shigenobu T, Takahashi Y, Masugi Y, Hanawa R, Matsushita H, Tajima A, Kuroda H. Micropapillary Predominance Is a Risk Factor for Brain Metastasis in Resected Lung Adenocarcinoma. Clin Lung Cancer. S1525-7304(21)00085-1. 2021
- Sato Y, Mori K, Hirano K, Yagi K, Kobayashi Y, Nagaoka K, Hosoi A, Matsushita H, Kakimi K, Seto Y. Adoptive γδT-cell transfer alone or combined with chemotherapy for the treatment of advanced esophageal cancer. Cytotherapy. 23(5):423-432. 2021
- Takahashi Y, Suzuki S, Hamada K, Nakada T, Oya Y, Sakakura N, Matsushita H, Kuroda H. Sarcopenia is poor risk for unfavorable short- and long-term outcomes in stage I non-small cell lung cancer. Ann Transl Med. 9(4):325. 2021
- Matsui T, Takahashi Y, Nakada T, Matsushita H, Oya Y, Sakakura N, Kuroda H. Efficacy of Xenon Light With Indocyanine Green for Intersegmental Visibility in Thoracoscopic Segmentectomy. J Surg Res.259:39-46. 2021
- Yabuno A, Matsushita H, Hamano T, Tan TZ, Shintani D, Fujieda N, Tan DSP, Huang RY, Fujiwara K, Kakimi K, Hasegawa K. Identification of serum cytokine clusters associated with outcomes in ovarian clear cell carcinoma. .Sci Rep. 10(1):18503. 2020
- Sato Y, Wada I, Odaira K, Hosoi A, Kobayashi Y, Nagaoka K, Karasaki T, Matsushita H, Yagi K, Yamashita H, Fujita M, Watanabe S, Kamatani T, Miya F, Mineno J, Nakagawa H, Tsunoda T, Takahashi S, Seto Y, Kakimi K.Integrative immunogenomic analysis of gastric cancer dictates novel immunological classification and the functional status of tumor-infiltrating cells. Clin Transl Immunology. 9(10):e1194. 2020
- Kakimi K, Matsushita H, Masuzawa K, Karasaki T, Kobayashi Y, Nagaoka K, Hosoi A, Ikemura S, Kitano K, Kawada I, Manabe T, Takehara T, Ebisudani T, Nagayama K, Nakamura Y, Suzuki R, Yasuda H, Sato M, Soejima K, Nakajima JAdoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory non-small-cell lung cancer: a multicenter, open-label, single-arm, phase 2 study. J Immunother Cancer.;8(2):e001185. 2020
- Matsushita H, Hasegawa K, Oda K, Yamamoto S, Asada K, Karasaki T, Yabuno A, Nishijima A, Nejo T, Kobayashi Y, Sato S, Ikeda Y, Miyai M, Takahashi Y, Yamaguchi R, Fujiwara K, Aburatani H, Kakimi K. Neoantigen load and HLA-class I expression identify a subgroup of tumors with a T cell-inflamed phenotype and favorable prognosis in homologous recombination-proficient high-grade serous ovarian carcinoma. J Immunother Cancer. 8(1):e000375. 2020
- Kobayashi Y, Yamada D, Kawai T, Sato Y, Teshima T, Yamada Y, Nakamura M, Suzuki M, Matsumoto A, Nakagawa T, Hosoi A, Nagaoka K, Karasaki T, Matsushita H, Kume H, Kakimi K. Different immunological effects of the molecular targeted agents sunitinib, everolimus and temsirolimus in patients with renal cell carcinoma. Int J Oncol. 56(4):999-1013. 2020
- Nejo T, Matsushita H, Karasaki T, Nomura M, Saito K, Tanaka S, Takayanagi S, Hana T, Takahashi S, Kitagawa Y, Koike T, Kobayashi Y, Nagae G, Yamamoto S, Ueda H, Tatsuno K, Narita Y, Nagane M, Ueki K, Nishikawa R, Aburatani H, Mukasa A, Saito N, Kakimi K. Reduced Neoantigen Expression Revealed by Longitudinal Multiomics as a Possible Immune Evasion Mechanism in Glioma. Cancer Immunol Res. 7(7):1148-1161. 2019
- Ohue Y, Kurose K, Karasaki T, Isobe M, Yamaoka T, Futami J, Irei I, Masuda T, Fukuda M, Kinoshita A, Matsushita H, Shimizu K, Nakata M, Hattori N, Yamaguchi H, Fukuda M, Nozawa R, Kakimi K, Oka M. Serum Antibody Against NY-ESO-1 and XAGE1 Antigens Potentially Predicts Clinical Responses to Anti-Programmed Cell Death-1 Therapy in NSCLC. J Thorac Oncol. 14(12):2071-2083. 2019
- Emoto K, Eguchi T, Tan KS, Takahashi Y, Aly RG, Rekhtman N, Travis WD, Adusumilli PS. Expansion of the Concept of Micropapillary Adenocarcinoma to Include a Newly Recognized Filigree Pattern as Well as the Classical Pattern Based on 1468 Stage I Lung Adenocarcinomas. J Thorac Oncol 14(11):1948-1961. 2019
- Aly RG, Rekhtman N, Li X, Takahashi Y, Eguchi T, Tan KS, Rudin CM, Adusumilli PS, Travis WD. Spread Through Air Spaces (STAS) Is Prognostic in Atypical Carcinoid, Large Cell Neuroendocrine Carcinoma, and Small Cell Carcinoma of the Lung. J Thorac Oncol 14(9):1583-1593. 2019
- Takahashi Y, Suzuki S, Matsutani N, Kawamura M. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the evaluation of clinically node-negative non-small cell lung cancer. Thorac Cancer 10(3):413-420. 2019
- Kataoka K, Miyoshi H, Sakata S, Dobashi A, Couronne L, Kogure Y, Sato Y, Nishida K, Gion Y, Shiraishi Y, Tanaka H, Chiba K, Watatani Y, Kakiuchi N, Shiozawa Y, Yoshizato T, Yoshida K, Makishima H, Sanada M, Onozawa M, Teshima T, Yoshiki Y, Ishida T, Suzuki K, Shimada K, Tomita A, Kato M, Ota Y, Izutsu K, Demachi-Okamura A, Akatsuka Y, Miyano S, Yoshino T, Gaulard P, Hermine O, Takeuchi K, Ohshima K, Ogawa S. Frequent structural variations involving programmed death ligands in Epstein-Barr virus-associated lymphomas. Leukemia. 33(7):1687-1699. 2019
- Ohta R, Demachi-Okamura A, Akatsuka Y, Fujiwara H, Kuzushima K. Improving TCR affinity on 293T cells. J Immunol Methods. 466:1-8. 2019
- Imai Y, Hasegawa K, Matsushita H, Fujieda N, Sato S, Miyagi E, Kakimi K, Fujiwara K. Expression of multiple immune checkpoint molecules on T cells in malignant ascites from epithelial ovarian carcinoma. Oncol Lett. 15(5):6457-6468. 2018
- Yamamoto M, Nomura S, Hosoi A, Nagaoka K, Iino T, Yasuda T, Saito T, Matsushita H, Uchida E, Seto Y, Goldenring JR, Kakimi K, Tatematsu M, Tsukamoto T. Established gastric cancer cell lines transplantable into C57BL/6 mice demonstrate FGFR4 promotion of tumor growth. Cancer Sci. 109(5):1480-1492. 2018.
- Nagaoka K, Hosoi A, Iino T, Morishita Y, Matsushita H, Kakimi K. Dendritic cell vaccine induces antigen-specific CD8+ T cells that are metabolically distinct from those of peptide vaccine and is well-combined with PD-1 checkpoint blockade. Oncoimmunology. 7(3):e1395124. 2017
- Hosoi A, Takeda K, Nagaoka K, Iino T, Matsushita H, Ueha S, Aoki S, Matsushima K, Kubo M, Morikawa T, Kitaura K, Suzuki R, Kakimi K. Increased diversity with reduced “diversity evenness” of tumor infiltrating T-cells for the successful cancer immunotherapy. Sci Rep. 8(1):1058. 2018
- Hoshikawa M, Aoki T, Matsushita H, Karasaki T, Hosoi A, Odaira K, Fujieda N, Kobayashi Y, Kambara K, Ohara O, Arita J, Hasegawa K, Kakimi K, Kokudo N.NK cell and IFN signatures are positive prognostic biomarkers for resectable pancreatic cancer. Biochem Biophys Res Commun. 495(2):2058-2065. 2018
- Matsushita H, Hasegawa K, Oda K, Yamamoto S, Nishijima A, Imai Y, Asada K, Ikeda Y, Karasaki T, Fujiwara K, Aburatani H, Kakimi K. The frequency of neoantigens per somatic mutation rather than overall mutational load or number of predicted neoantigens per se is a prognostic factor in ovarian clear cell carcinoma. Oncoimmunology. 6(8):e1338996. 2017
- Aoki T, Matsushita H, Hoshikawa M, Hasegawa K, Kokudo N, Kakimi K. Adjuvant combination therapy with gemcitabine and autologous γδ T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 19(4):473-485. 2017
- Karasaki T, Nagayama K, Kuwano H, Nitadori JI, Sato M, Anraku M, Hosoi A, Matsushita H, Morishita Y, Kashiwabara K, Takazawa M, Ohara O, Kakimi K, Nakajima J. An Immunogram for the Cancer-Immunity Cycle: Towards Personalized Immunotherapy of Lung Cancer. J Thorac Oncol. 12(5):791-803. 2017
- Karasaki T, Nagayama K, Kuwano H, Nitadori JI, Sato M, Anraku M, Hosoi A, Matsushita H, Takazawa M, Ohara O, Nakajima J, Kakimi K. Prediction and prioritization of neoantigens: integration of RNA sequencing data with whole-exome sequencing. Cancer Sci. 108(2):170-177. 2017
- Odaira K, Kimura SN, Fujieda N, Kobayashi Y, Kambara K, Takahashi T, Izumi T, Matsushita H, Kakimi K. CD27(-)CD45(+) γδ T cells can be divided into two populations, CD27(-)CD45(int) and CD27(-)CD45(hi) with little proliferation potential. Biochem Biophys Res Commun. 478(3):1298-303. 2016
- Matsushita H, Sato Y, Karasaki T, Nakagawa T, Kume H, Ogawa S, Homma Y, Kakimi K. Neoantigen load, antigen presentation machinery, and immune signatures determine prognosis in clear cell renal cell carcinoma. Cancer Immunol Res. 4(5): 463-71. 2016
- Makise N, Morikawa T, Nakagawa T, Ichimura T, Kawai T, Matsushita H, Kakimi K, Kume H, Homma Y, Fukayama M. MAGE-A expression, immune microenvironment, and prognosis in upper urinary tract carcinoma. Hum Pathol. 50:62-9. 2016
- Karasaki T, Nagayama K, Kawashima M, Hiyama N, Murayama T, Kuwano H, Nitadori JI, Anraku M, Sato M, Miyai M, Hosoi A, Matsushita H, Kikugawa S, Matoba R, Ohara O, Kakimi K, Nakajima J. Identification of Individual Cancer-Specific Somatic Mutations for Neoantigen-Based Immunotherapy of Lung Cancer. J Thorac Oncol. 11(3):324-333. 2015
- Matsushita H, Hosoi A, Ueha S, Abe J, Fujieda N, Tomura M, Maekawa R, Matsushima K, Ohara O, Kakimi K. Cytotoxic T lymphocytes block tumor growth both by lytic activity and IFNγ-dependent cell-cycle arrest. Cancer Immunol Res. Jan;3(1):26-36. 2015
- Futami J, Nonomura H, Kido M, Niidoi N, Fujieda N, Hosoi A, Fujita K, Mandai K, Atago Y, Kinoshita R, Honjo T, Matsushita H, Uenaka A, Nakayama E, Kakimi K. Sensitive Multiplexed Quantitative Analysis of Autoantibodies to Cancer Antigens with Chemically S-Cationized Full-Length and Water-Soluble Denatured Proteins. Bioconjug Chem. 26(10):2076-84. 2015
- Miyai M, Eikawa S, Hosoi A, Iino T, Matsushita H, Isobe M, Uenaka A, Udono H, Nakajima J, Nakayama E, Kakimi K. Detection and Tracking of NY-ESO-1-Specific CD8+ T Cells by High-Throughput T Cell Receptor β (TCRB) Gene Rearrangements Sequencing in a Peptide-Vaccinated Patient. PLoS One. Aug 20;10(8):e0136086. 2015
- Hirano K, Hosoi A, Matsushita H, Iino T, Ueha S, Matsushima K, Seto Y, Kakimi K. The nitric oxide radical scavenger carboxy-PTIO reduces the immunosuppressive activity of myeloid-derived suppressor cells and potentiates the antitumor activity of adoptive cytotoxic T lymphocyte immunotherapy. Oncoimmunology. 4(8). 2015.
- Futami J, Fujiyama H, Kinoshita R, Nonomura H, Honjo T, Tada H, Matsushita H, Abe Y, Kakimi K. Denatured Mammalian protein mixtures exhibit unusually high solubility in nucleic Acid-free pure water. PLoS One. 9(11):e113295. 2014
- Yamada D, Matsushita H, Azuma T, Nakagawa T, Nagata M, Yamada Y, Suzuki M, Fujimura T, Fukuhara H, Kume H, Homma Y, Kakimi K. Granulocyte macrophage colony-stimulating factor as a predictor of the response of metastatic renal cell carcinoma to tyrosine kinase inhibitor therapy. Mol Clin Oncol. 2(6):1023-1027. 2014
- Matsushita H, Enomoto H, Kume H, Nakagawa T, Fukuhara H, Suzuki M, Fujimura T, Homma Y and Kakimi K. A pilot study of autologous tumor lysate-loaded dendritic cell vaccination combined with sunitinib for metastatic renal cell carcinoma. Journal for Immunotherapy of Cancer 2:30. 2014
- Kawai T, Enomoto Y, Morikawa T, Matsushita H, Kume H, Fukayama M, Yamaguchi H, Kakimi K, Homma Y. High expression of heat shock protein 105 predicts a favorable prognosis for patients with urinary bladder cancer treated with radical cystectomy.Mol Clin Oncol. 2(1):38-42. 2014
- Wada I, Matsushita H, Noji S, Mori K, Yamashita H, Nomura S, Shimizu N, Seto Y, Kakimi K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 3(2):362-75. 2014
- Wada H, Isobe M, Kakimi K, Mizote Y, Eikawa S, Sato E, Takigawa N, Kiura K, Tsuji K, Iwatsuki K, Yamasaki M, Miyata H, Matsushita H, Udono H, Seto Y, Yamada K, Nishikawa H, Pan L, Venhaus R, Oka M, Doki Y, Nakayama E.Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil OK-432 and montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. J Immunother. 37(2):84-92. 2014
- Ichimura T, Morikawa T, Kawai T, Nakagawa T, Matsushita H, Kakimi K, Kume H, Ishikawa S, Homma Y, Fukayama M. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann Surg Oncol. Jun;21(6):2105-12. 2014
- Hosoi A, Matsushita H, Shimizu K, Fujii S, Ueha S, Abe J, Kurachi M, Maekawa R, Matsushima K, Kakimi K. Adoptive cytotoxic T lymphocyte therapy triggers a counter-regulatory immunosuppressive mechanism via recruitment of myeloid-derived suppressor cells. Int J Cancer. Apr 15;134(8):1810-22. 2014
- Izumi T, Kondo M, Takahashi T, Fujieda N, Kondo A, Tamura N, Murakawa T, Nakajima J, Matsushita H, Kakimi K. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy. Apr;15(4):481-91. 2013
- Shimizu K, Mizuno T, Shinga J, Asakura M, Kakimi K, Ishii Y, Masuda K, Maeda T, Sugahara H, Sato Y, Matsushita H, Nishida K, Hanada K, Dorrie J, Schaft N, Bickham K, Koike H, Ando T, Nagai R, Fujii S. Vaccination with antigen-transfected, NKT cell ligand-loaded, human cells elicits robust in situ immune responses by dendritic cells.Cancer Res. Jan 1;73(1):62-73. 2013
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 482(7385): 400-4. 2012
- Noji S, Hosoi A, Takeda K, Matsushita H, Morishita Y, Seto Y, Kakimi K. Targeting spatiotemporal expression of CD137 on tumor-infiltrating cytotoxic T lymphocytes as a novel strategy for agonistic antibody therapy. J Immunother. Jul;35(6):460-72. 2012
- Kondo M, Izumi T, Fujieda N, Kondo A, Morishita T, Matsushita H, Kakimi K. Expansion of Human Peripheral Blood γδ T Cells using Zoledronate. J Vis Exp. Sep 9 ;(55) pii:3182. 2011
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 208(10):1989-2003. 2011
- Sakamoto M, Nakajima J, Murakawa T, Fukami T, Yoshida Y, Murayama T, Takamoto S, Matsushita H, Kakimi K. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδTcells: a phase I clinical study. J Immunother. Mar;34(2):202-11. 2011
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, and Murphy KM. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 322, 1097-1100. 2008
- Ishida T, Obata Y, Ohara N, Matsushita H, Sato S, Uenaka A, Saika T, Miyamura T, Chayama K, Nakamura Y, Wada H, Yamashita T, Morishima T, Old LJ, Nakayama E .Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 8:15. 2008
Education & Training
We are always recruiting researchers from inside or outside of Japan. To develop effective cancer immunotherapies, we would like to work with young students or postdocs who have shown research abilities and the ability to perform in collaborative projects within a team. Good communication skills and an enthusiastic personality are expected.
Our laboratory is a part of the Nagoya University Graduate School of Medicine. Please follow this link:Graduate Programs

