Division of Pathophysiology
Introduction
At the Division of Pathophysiology, we are studying how cancer cells emerge and progress to threaten life. Using genetically engineered and other mouse models of solid cancers, we are currently focusing on the following:
- clarifying the roles of the tumor microenvironment in cancer formation and progression.
- elucidating the molecular mechanisms of metastasis.
- unraveling the pathophysiology of cancer cachexia.
Research topics
Cancer is a systemic disease. Heterotypic interactions between cancer cells and non-cancer stromal cells in the tumor microenvironment play essential roles in cancer progression. Metastasis, the spread of cancer cells from primary sites to different parts of the body, and cachexia, a wasting syndrome characterized by progressive loss of skeletal muscle mass, represent systemic changes that are major causes of cancer mortality.Using genetically engineered and other mouse models of colorectal solid cancers, we are currently focusing on the following: (1) clarifying roles of the tumor microenvironment in cancer formation and progression; (2) elucidating the molecular mechanisms of metastasis; and (3) unraveling the pathophysiology of cancer cachexia.
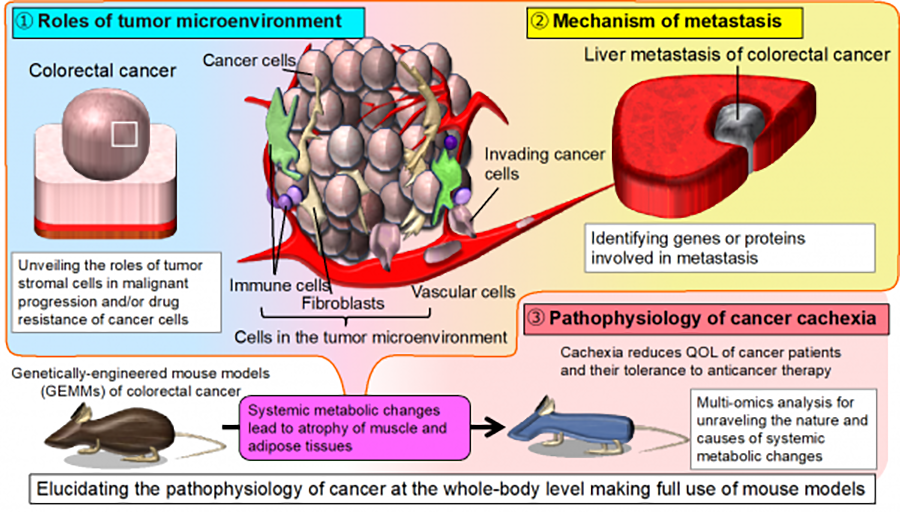
1)Clarifying the roles of tumor microenvironment in cancer formation and progression
Cancer tissue is composed of cancer cells and host cells in the stroma, particularly fibroblasts, immune cells such as lymphocytes, and macrophages, as well as vascular cells. This so-called tumor microenvironment has been shown to play important roles in the growth, invasion, and metastasis of cancer cells. We are attempting to clarify its various influences at the whole-body level to identify novel strategies for cancer treatment.
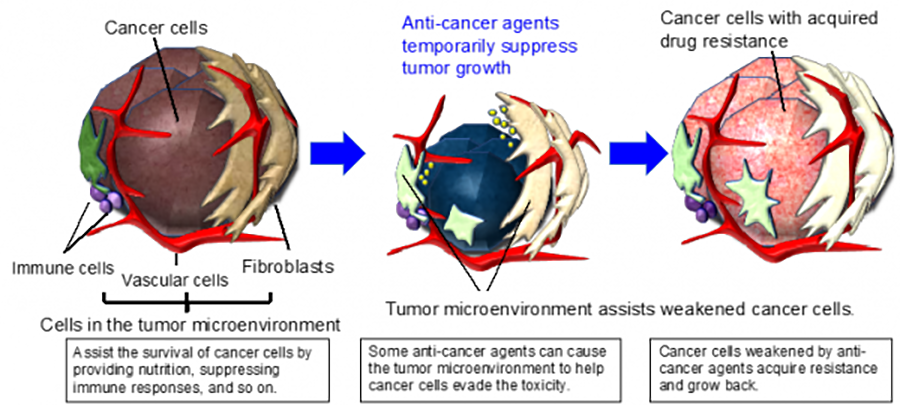
Recent developments in molecularly targeted anti-cancer agents have greatly improved the treatment of certain types of cancer. However, resistance to such agents is often acquired, leading to relapse. We found that colorectal cancer cells can acquire resistance to a molecularly targeted agent using normal stromal cells in the tumor microenvironment (Figure 2, https://www.ncbi.nlm.nih.gov/pubmed/28759045).
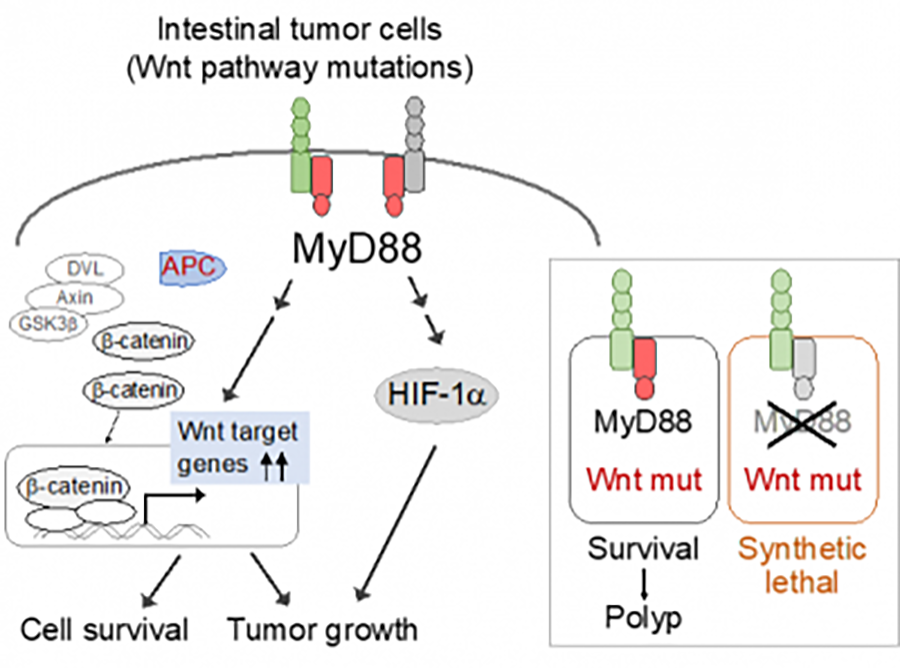
We have also recently discovered that Myd88, an important adaptor protein involved in TLR and IL-1 signaling, is essential for colorectal carcinogenesis. Thus, MyD88 loss proved synthetic lethal with mutational activation of Wnt/β-catenin signaling in intestinal tumor epithelial cells (Figure 3, https://pubmed.ncbi.nlm.nih.gov/33177648/). Inhibition of MyD88 signaling may be a novel therapeutic strategy for colorectal cancers featuring mutations in Wnt/β-catenin signaling pathways.
2)Elucidating the molecular mechanisms of metastasis
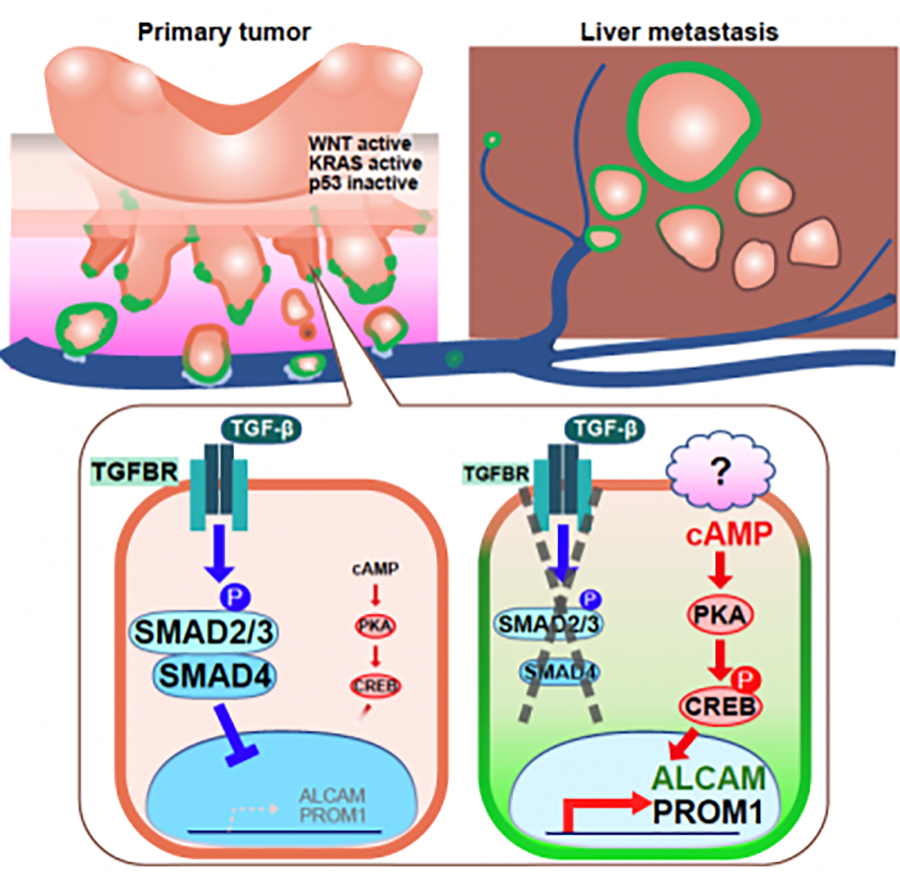
The prognosis of colorectal cancer patients with metastases is poor. Recent studies suggest that cancer stem cells are implicated in metastasis, drug resistance, and recurrence. Several cell-surface proteins have been proposed as colorectal cancer stem cell markers, but their involvement is not fully understood. Signaling pathways regulating colorectal cancer stemness are also largely unknown.
We have generated a genetically-engineered CKPS mouse model of sporadic colorectal cancer featuring spontaneous distant metastasis, by introducing mutations into four genes, Ctnnb1 encoding β-catenin, Kras, Tp53, and Smad4, in intestinal epithelial cells. We found elevated expression of ALCAM and PROM1, known markers of colorectal cancer stem cells, in metastatic adenocarcinomas of CKPS mice and demonstrated their functional importance in stemness and metastatic potential (Figure 4, https://pubmed.ncbi.nlm.nih.gov/36066360/). We further showed that expression of ALCAM and PROM1 is regulated negatively by the TGFβ/SMAD4 and positively by the cAMP-PKA-CREB pathway (Figure 4). These results indicate that the latter may be a potential therapeutic target for colorectal cancer.
We also conduct functional screening for regulators of colorectal cancer metastasis by shRNA library and PiggyBac transposon mutagenesis approaches. Using an shRNA screen, we recently identified HNRNPLL encoding a pre-mRNA splicing factor as a colorectal cancer metastasis suppressor gene. We found that HNRNPLL regulates alternative splicing of the pre-mRNA encoding CD44, an essential molecule involved in colorectal cancer metastasis, during the epithelial-mesenchymal transition (EMT) (Figure 5, https://www.ncbi.nlm.nih.gov/pubmed/28360095).
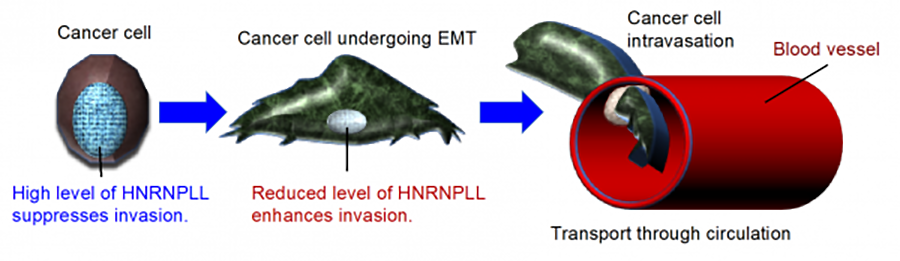
3)Unraveling the pathophysiology of cancer cachexia
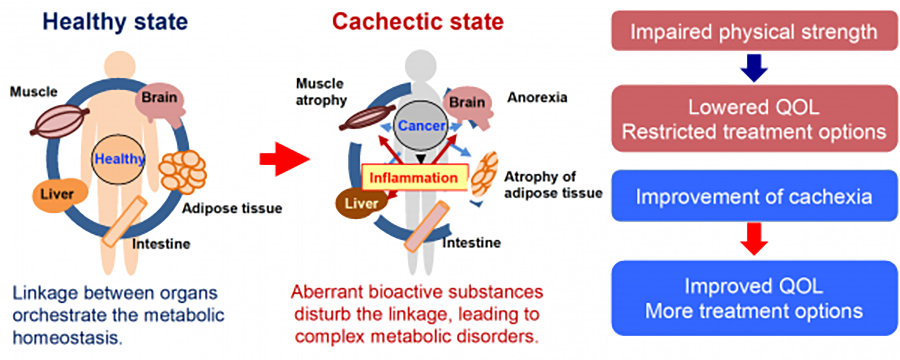
Cachexia, a wasting syndrome characterized by the progressive loss of skeletal muscle mass and body weight, occurs in up to 80% of advanced cancer patients and is considered a direct cause of ~20% of cancer-related deaths. Although increasing knowledge indicates that cancer cachexia is a complex whole-body metabolic disorder, its pathophysiology remains poorly understood. To explore the systemic metabolic changes that contribute to the pathogenesis of cancer cachexia, we are conducting multi-omics analysis (metabolomics, transcriptomics, and proteomics) of metabolic organs in mouse models of cancer featuring cachexia.
Members
2) Pathophysiology of cancer cachexia
Other members
- Yoshiko Goto, Research Assistant
- Kyoko Kobori, Research Assistant
- Yu Li, Visiting Trainee
- Ensei Gyu, Visiting Trainee
- Chika Niwa, Visiting Trainee
- Hiromi Tamaki, Administrative assistant
Alumni since 2011
- Mark van Boxtel
- Yukie Fuma
- Adam Douglas
- Ryota Mitsuya, Japanese Red Cross Nagoya Daini Hospital
- Aki Kojima
- Yumiko Ito, Department of Pathology and Molecular Diagnostics, Aichi Cancer Center Hospital
- Miyako Tsuda
- Yuki Takada
- Florence Orim
- Hosne Ara, Department of Cellular Biology and Anatomy, Louisiana State University Health Sciences
- Keiichiro Sakuma, Professor, Department of Food Science and Nutrition, Graduate School of Agriculture, Kindai University
- Emi Mishiro, Center chief, Designated Lecturer, Molecular Structure Center, Institute of Transformative Biomolecules (ITbM), Nagoya University
Publications
Original articles
- Fujishita T, Kojima Y, Kajino-Sakamoto R, Mishiro-Sato E, Shimizu Y, Hosoda W, Yamaguchi R, Taketo MM, Aoki M.: The cAMP/PKA/CREB and TGF-β/SMAD4 pathways regulate stemness and metastatic potential in colorectal cancer cells. Cancer Res. 82(22): 4179-4190, 2022. doi: 10.1158/0008-5472.CAN-22-1369. (PMID: 36066360)
- Sakuma K, Sasaki E, Hosoda Y, Komori K, Shimizu Y, Yatabe Y, Aoki M.: MYB mediates downregulation of the colorectal cancer metastasis suppressor HNRNPLL during epithelial-mesenchymal transition. Cancer Sci. 112(9): 3846-3855, 2021. doi: 10.1111/cas.15069. (PMID: 34286904)
- Tanigawa S, Fujita M, Mayama C, Ando S, Ii H, Kojima Y, Fujishita T, Aoki M, Takauchi H, Yamanaka T, Takahashi Y, Hashimoto N, Nakata S.: Inhibition of Gli2 suppresses tumorigenicity in glioblastoma stem cells derived from a de novo murine brain cancer model. Cancer Gene Ther, 28 (12): 1339-1352, 2021, Jan 7, doi: 10.1038/s41417-020-00282-5. (PMID: 33414520)
- Kajino-Sakamoto R, Fujishita T, Taketo MM, Aoki M.: Synthetic lethality between MyD88 loss and mutations in Wnt/β-catenin pathway in intestinal tumor epithelial cells. Oncogene. 40(2): 408-420, 2021. doi: 10.1038/s41388-020-01541-3. (PMID: 33177648)
- Kojima Y, Kondo Y, Fujishita T, Mishiro-Sato E, Kajino-Sakamoto R, Taketo MM, Aoki M. Stromal iodothyronine deiodinase 2 (DIO2) promotes the growth of intestinal tumors in ApcΔ716 mutant mice. Cancer Sci. 110(8):2520-2528, 2019. doi: 10.1111/cas.14100. (PMID: 31215118)
- Hijioka S, Sakuma K, Aoki M, Mizuno N, Kuwahara T, Okuno N, Hara K, Yatabe Y.: Clinical and in vitro studies of the correlation between MGMT and the effect of streptozocin in pancreatic NET. Cancer Chemother Pharmacol, 83(1):43-52, 2019. doi: 10.1007/s00280-018-3700-y. (PMID: 30310970)
- Matsushita A, Sato T, Mukai S, Fujishita T, Mishiro E, Aoki M, Hasegawa Y, Sekodo Y. TAZ activation by Hippo pathway dysregulation induces cytokine gene expression and promotes mesothelial cell transformation. Oncogene, 38(11):1966-1978, 2019. doi: 10.1038/s41388-018-0417-7. (PMID: 30401981)
- Maeda A, Irie K, Ando H, Hasegawa A, Taniguchi H, Kadowaki S, Muro K, Tajika M, Aoki M, Inaguma K, Kajita M, Fujimura A, Fukushima S.: Associations among regorafenib concentrations, severe adverse reactions, and ABCG2 and OATP1B1 polymorphisms. Cancer Chemother Pharmacol, 83(1):107-113, 2019. doi: 10.1007/s00280-018-3710-9. (PMID: 30368586)
- Sakuma K, Sasaki E, Kimura K, Komori K, Shimizu Y, Yatabe Y, Aoki M. HNRNPLL stabilizes mRNAs for DNA replication proteins and promotes cell cycle progression in colorectal cancer cells. Cancer Sci. 109(8):2458-2468, 2018. doi: 10.1111/cas.13660. (PMID: 29869816)
- Satoh K, Yachida S, Sugimoto M, Oshima M, Nakagawa T, Akamoto S, Tabata S, Saitoh K, Kato K, Sato S, Igarashi K, Aizawa Y, Kajino-Sakamoto R, Kojima Y, Fujishita T, Enomoto A, Hirayama A, Ishikawa T, Taketo MM, Kushida Y, Haba R, Okano K, Tomita M, Suzuki Y, Fukuda S, Aoki M, Soga T. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. PNAS, 114(31): 8289-8294, 2017. doi: 10.1073/pnas.1620915114. (PMID: 28716939)
- Sakuma K, Sasaki E, Kimura K, Komori K, Shimizu Y, Yatabe Y, Aoki M.: HNRNPLL, a newly identified colorectal cancer metastasis suppressor, modulates alternative splicing of CD44 during epithelial-mesenchymal transition. Gut. 67(6): 1103-1111, 2018. doi: 10.1136/gutjnl-2016-312927. (PMID: 28360095)
- Cao X, Kajino-Sakamoto R, Doss A, Aballay A. Distinct Roles of Sensory Neurons in Mediating Pathogen Avoidance and Neuropeptide-Dependent Immune Regulation. Cell Rep. 2017 Nov 7;21(6):1442-1451. doi: 10.1016/j.celrep.2017.10.050.
- Maeda A, Ando H, Ura T, Muro K, Aoki M, Saito K, Kondo E, Takahashi S, Ito Y, Mizuno Y, Fujimura A. Differences in urinary renal failure biomarkers in cancer patients initially treated with cisplatin. Anticancer Res. 37(9): 5235-5339, 2017. doi: 10.21873/anticanres.11947. (PMID: 28870959)
- Fujishita T, Kojima Y, Kajino-Sakamoto, R, Taketo, MM, Aoki M. Tumor microenvironment confers mTOR inhibitor resistance in invasive intestinal adenocarcinoma. Oncogene. 36(46): 6480-6489, 2017. doi: 10.1038/onc.2017.242. (PMID: 28759045)
- Maeda A, Ando H, Ura T, Komori A, Hasegawa A, Taniguchi H, Kadowaki S, Muro K, Tajika M, Kobara M, Matsuzaki M, Hashimoto N, Maeda M, Kojima Y, Aoki M, Kondo E, Mizutani A, Fujimura A. Association between and polymorphisms and adverse drug reactions to regorafenib: A preliminary study. Int J Clin Pharmacol Ther. 55(5): 409-415, 2017. doi: 10.5414/CP202788. (PMID: 28157071)
- Hashimoto K, Simmons AN, Kajino-Sakamoto R, Tsuji Y, Ninomiya-Tsuji J. TAK1 regulates the Nrf2 antioxidant system through modulating p62/ SQSTM1. Antioxid Redox Signal. 25: 953-964, 2016 (PMID: 27245349)
- Hijiya N, Tsukamoto Y, Nakada C, Tung N, Kai T, Matsuura K, Shibata K, Inomata M, Uchida T, Tokunaga A, Amada K, Shirao K, Yamada Y, Mori H, Takeuchi I, Seto M, Aoki M, Takekawa M, Moriyama M.: Genomic loss of DUSP4 contributes to the progression of intraepithelial neoplasm of pancreas to invasive carcinoma. Cancer Res. 76:2612-2625, 2016 (PMID: 26941286)
- Simmons AN, Kajino-Sakamoto R, Ninomiya-Tsuji J.: TAK1 regulates Paneth cell integrity partly through blocking necroptosis. Cell Death Dis. 7:e2196, 2016 (PMID: 27077812)
- Fujishita T, Kajino-Sakamoto R, Kojima Y, Taketo MM, Aoki M.: Antitumor activity of the MEK inhibitor trametinib on intestinal polyp formation in ApcΔ716 mice involves stromal COX-2. Cancer Sci, 106: 692-699, 2015 (PMID:25855137)
- Hirai H, Fujishita T, Kurimoto K, Miyachi H, Kitano S, Inamoto S, Itatani Y, Saitou M, Maekawa T,Taketo MM.: CCR1-mediated accumulation of myeloid cells in the liver microenvironment promoting mouse colon cancer metastasis. Clin Exp Metastasis, 31: 977-989, 2014 (PMID: 25326065)
- Patnode M, Yu S-Y, Cheng C-W, Ho M-Y, Tegesjo L, Sakuma K, Uchimura K, Khoo K-H, Kannagi R, Rosen S.: KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin dependent lymphocyte homing. Glycobiology, 23: 381-394, 2013. (PMID: 23254996)
- Itatani Y, Kawada K, Fujishita T, Kakizaki F, Hirai H, Matsumoto T, Iwamoto M, Inamoto S, Hatano E, Hasegawa S, Maekawa T, Uemoto S, Sakai Y, Taketo MM.: Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology, 145: 1064-1075, 2013. (PMID: 23891973)
- Sakuma K, Furuhashi T, Kondo S, Yabe U, Ohmori K, Ito H, Aoki M, Morita A, Kannagi R.: Sialic acid cyclization of human Th homing receptor glycan associated with recurrent exacerbations of atopic dermatitis. J Dermatol Sci, 68: 187-193, 2012. (PMID: 23088960)
- Sakuma K, Aoki M, Kannagi R.: Transcription factors c-MYC and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc Natl Acad Sci U S A, 109: 7776-7781, 2012. (PMID: 22547830)
- Miyazaki K, Sakuma K, Kawamura Y, Izawa M, Ohmori K, Mitsuki M, Yamaji T, Hashimoto Y, Suzuki A, Saito Y, Dohi T, Kannagi R.: Colonic epithelial cells express specific ligands for mucosal macrophage immunosuppressive receptors siglec-7 and -9. J Immunol, 188: 4690-700, 2012. (PMID: 22467657)
- Sakuma K, Chen GY, Aoki M, Kannagi R.: Induction of 6-sulfated glycans with cell adhesion activity via T-bet and GATA-3 in human helper T cells. Biochim Biophys Acta, 1820: 841-848, 2012. (PMID: 22446378)
- Fujishita T, Aoki M, Taketo MM.: JNK signaling promotes intestinal tumorigenesis through activation of mTOR complex 1 in ApcΔ716 mice. Gastroenterology, 140:1556-1563, 2011. (PMID: 21320501)
- Aoki K, Kakizaki F, Sakashita H, Manabe T, Aoki M, Taketo MM.: Suppression of colonic polyposis by homeoprotein CDX2 through its nontranscriptional function that stabilizes p27Kip1. Cancer Res, 71: 593-602, 2011. (PMID: 21224344)
- Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hashida H, Takabayashi A, Sasaki M, Robine S, Itoh K, Yoshioka K, Kakizaki F, Kitamura T, Oshima M, Taketo MM.: Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell, 19: 125-137, 2011. (PMID: 21251616)
- Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science, 332: 729-732, 2011. (PMID: 21474712)
- Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kndoh S, Aoki M, Taketo MM.: Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci USA, 107: 13063-13068, 2010. (PMID: 20616008)
- Deguchi A, Miyoshi H, Kojima Y, Okawa K, Aoki M, Taketo MM.: LKB1 suppresses p21-activated kinase-1 (PAK1) by phosphorylation of Thr109 in the p21-binding domain. J Biol Chem, 285: 18282-18290, 2010. (PMID: 20400510)
- Kakizaki F, Aoki K, Miyoshi H, Carrasco N, Aoki M, Taketo MM.: CDX transcription factors positively regulate expression of Solute Carrier Family 5, Member 8 in the colonic epithelium. Gastroenterology, 138: 627-635, 2010. (PMID: 19900445)
- Kajino-Sakamoto R, Omori E, Nighot PK, Blikslager AT, Matsumoto K, Ninomiya-Tsuji J. TGF-beta-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J Immunol, 185: 4729-4727, 2010. (PMID: 20855879)
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A, 107: 20009-20014, 2010. (PMID: 21041659)
- Kitamura T, Biyajima K, Aoki M, Oshima M and Taketo MM. Matrix metalloproteinase 7 is required for tumor formation, but dispensable for invasion and fibrosis in SMAD4-deficient intestinal adenocarcinomas. Lab. Invest, 89: 98-105, 2009. (PMID: 19002110)
- Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. Reduced level of Smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology, 137: 629-638, 2009. (PMID: 19427313)
- Miyoshi H, Deguchi A, Nakau M, Kojima Y, Mori A, Oshima M, Aoki M, Taketo MM. Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1 mice. Cancer Sci, 100: 2046-2053, 2009. (PMID: 19671058)
- Morioka S, Omori E, Kajino T, Kajino-Sakamoto R, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase determines TRAIL sensitivity by modulating reactive oxygen species and cIAP. Oncogene, 28: 2257-2265, 2009. (PMID: 19421137)
- Kim JY, Kajino-Sakamoto R, Omori E, Jobin C, Ninomiya-Tsuji J. Intestinal epithelial-derived TAK1 signaling is essential for cytoprotection against chemical-induced colitis. PLoS One, 4: e4561, 2009. (PMID: 19234607)
Review articles
- Aoki, M and Fujishita, T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 407: 153-189, 2017. (PMID: 28550454)
- Aoki M, Taketo MM. Use of Genetically Engineered Mouse Models in Identification and Validation of Therapeutic Targets for Colon Cancer. in Targeting the Wnt Pathway in Cancer ed. Goss, Kahn. 2011. Springer, New York. (ISBN: 978-1-4419-8022-9)
- Fujishita T, Aoki M, Taketo MM. The role of mTORC1 pathway in intestinal tumorigenesis. Cell Cycle, 22: 3684-3687, 2009. (PMID: 19855161)
- Kannagi R, Sakuma K, Miyazaki K, Lim KT, Yusa A, Yin J, Izawa M. Altered expression of glycan genes in cancers induced by epigenetic silencing and tumor hypoxia: Clues in the ongoing search for new tumor markers. Cancer Sci, 101: 586-593, 2009. (PMID: 20085584)
Education & Training
Applications for graduate research are welcome from those who are dedicated to research and have strong interest in our projects. Our laboratory is part of the Nagoya University Graduate School of Medicine and Nagoya City University Graduate School of Pharmaceutical Sciences. Please refer to the following links for more information.
Nagoya University Graduate School of Medicine:
https://www.med.nagoya-u.ac.jp/medical_E/
Nagoya City University Graduate School of Pharmaceutical Sciences:
https://www.nagoya-cu.ac.jp/phar/english/

