Division of Cancer Biology
Introduction
Our goal is to identify genetic lesions and epigenetic alterations associated with the development and progression of human solid cancers and use this information to develop more effective approaches for their prevention, diagnosis, and treatment. Malignant mesothelioma (MM), which develops following asbestos exposure after a long latent period, is very aggressive and highly refractory to conventional therapeutic modalities such as chemotherapy, surgery, and radiation. Although combination chemotherapy of cisplatin and pemetrexed has been used for patients with advanced stage MM, immune checkpoint inhibitors have been shown to be effective for a subset of patients.
We have been studying the genetics and biology of MM cells using various approaches, including establishment of cell lines from patient tissues, comprehensive analysis of genetic and epigenetic alterations by next-generation sequencing, and in vitro and in vivo analysis of how altered cell signaling pathways which are often dysregulated in MM can confer malignant phenotypes on normal mesothelial cells. Based on these basic research results, we aim to develop new tools for new molecular targeted therapies against this very aggressive disease.
Research topics
Establishment and characterization of MM cell lines
We have established over 30 mesothelioma cell lines from Japanese patients. Using these cell lines, we conduct various in vitro and in vivo experiments to determine the molecular mechanisms by which mesothelial cells gain malignant phenotypes. These cell lines have been sequentially deposited at the RIKEN BioResource Research Center (Tsukuba, Japan) and we hope they will be of use to researchers worldwide in their mesothelioma research.
(Red, actin ; Green, PKM2)
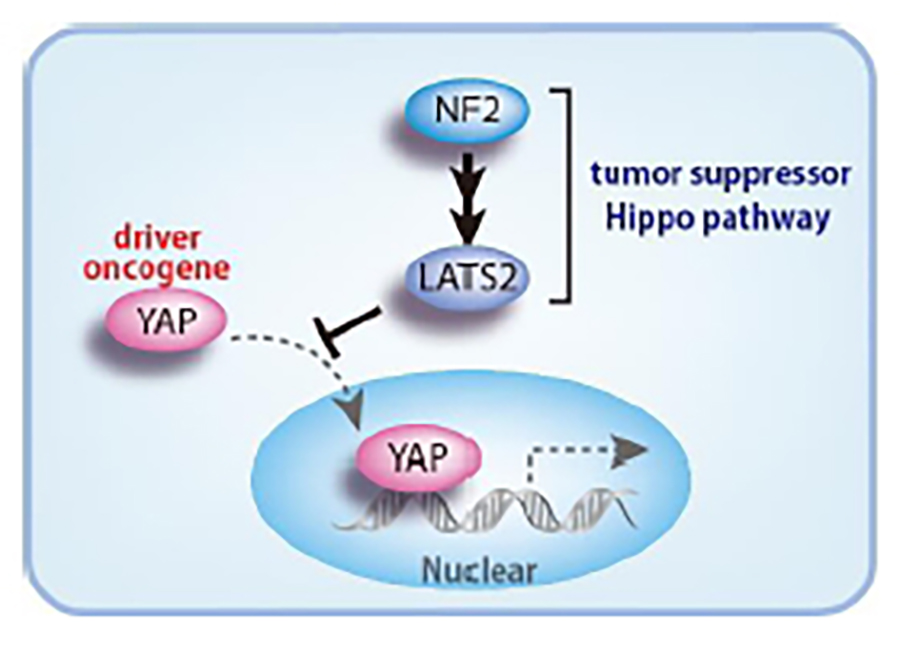
Elucidation of the NF2/merlin-Hippo signaling pathway in MM and drug development
Our group showed that the NF2 tumor suppressor gene, which is responsible for a familial cancer syndrome, neurofibromatosis type II, is highly mutated in MM cells. We also identified mutations in NF2 downstream genes including LATS2. The signaling pathway mediated by these gene products is known as the “Hippo signaling pathway,” in which aberrations activate transcriptional co-activators YAP and TAZ, which leads to TEAD family transcription factor activation. Our studies revealed constitutively activated transcription of genes encoding growth factors and cytokines, such as connective tissue growth factor (CTGF) and interleukin 1 beta (IL1B), by activation of YAP and TAZ, promote tumor progression. Collaborating with a pharmaceutical company, we have developed a new class inhibitor targeting TEAD which suppresses mesothelioma cell proliferation.
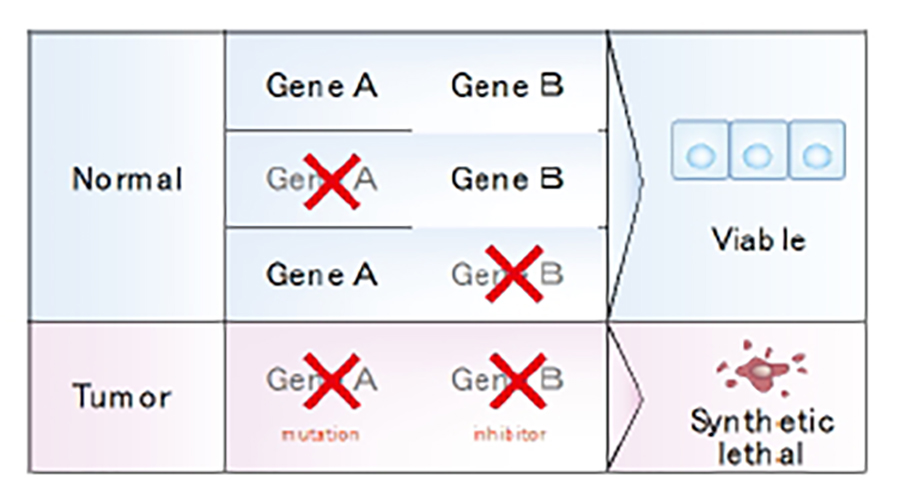
Development of molecular targeted drugs that induce synthetic lethality
The most frequently mutated genes in MM, BAP1, NF2, and CDKN2A/2B, are all tumor suppressors, and our challenge is to develop drugs that directly targeting these. Normal cells can sometimes remain viable when one gene (gene A) alone is perturbed, because another gene (gene B) can compensate. However, perturbation of both genes (A and B) simultaneously results in the loss of cell viability. This phenomenon is known as ‘synthetic lethality.’ Because the frequently mutated tumor suppressor genes identified in MM are not functional, drug development that leads to synthetic lethality is thought to be ideal for preferentially killing MM cells. We are attempting to identify genes that induce synthetic lethality in MM cells, especially examples susceptible to NF2 or LATS1/2 inactivation.
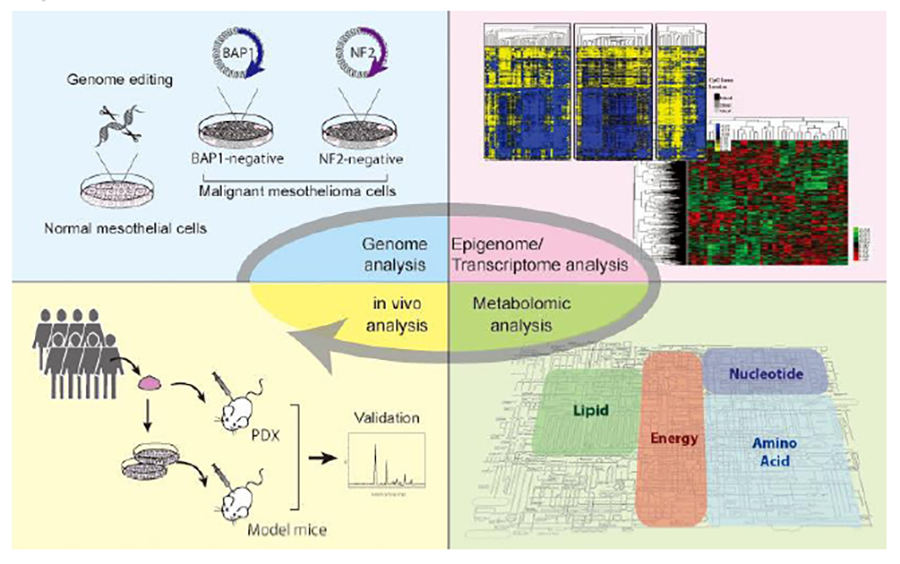
Comprehensive understanding of gene alterations, metabolites, and cell characteristics of MM and identification of novel drug targets for MM therapy
Our group found that driver mutations responsible for tumorigenesis occur in tumor suppressor genes in MM, with wide range genomic loss as a hallmark feature. Given that these alterations may cause distinctive metabolic reprogramming, MM cells may exhibit increased metabolic vulnerability. Moreover, genetic alterations may enhance the intrinsic characteristics of mesothelial cells, such as anoikis resistance and early onset epithelial-mesenchymal transition (EMT). We aim to explore these possibilities through comprehensive analyses of our established MM cell lines.
Members
1985~1988 Nagoya Ekisaikai Hospital. Medical Intern
1988~1992 Nagoya University Graduate School of Medicine, Doctoral course, Completed
1992~1997 Postdoctoral Fellow, University of Texas Southwestern Medical Center at Dallas
1997 Assistant Professor, University of Texas Southwestern Medical Center at Dallas
1998~2005 Assistant Professor, Department of Clinical Preventive Medicine, Nagoya Univ. School of Medicine
2005~ Present Incumbent
2013~ Present Aichi Cancer Center Research Institute, Vice Director (Additional post), Department of Cancer Genetics, Nagoya Univ. School of Medicine, Visiting Professor (Additional post)
2. Cellar characteristics of mesothelioma
3. Basic research to explore molecular targets in solid tumors
2006~2009 University of California, Los Angeles (UCLA), Postdoctoral fellow
2009~2016 Kitasato University School of Pharmaceutical sciences, Assistant Professor
2016~ Present Incumbent
2. Elucidation of the molecular basis for mesothelioma
3. Target identification and validation for novel drug discovery
2010 Kobe University Graduate school of Medicine, Master course, Completed
2013 Osaka University Graduate School of Frontier Biosciences, Doctoral course, Completed
2013~2016 Osaka University Research Institute for Microbial Diseases, Postdoctoral fellow
2016~ present Incumbent
2. Molecular and cellular biological analysis of mesothelioma
Publications
Original articles
2024
- Hirai S, Yamada T, Katayama Y, Ishida M, Kawachi H, Matsui Y, Nakamura R, Morimoto K, Horinaka M, Sakai T, Sekido Y, Tokuda S, Takayama K. Effects of Combined Therapeutic Targeting of AXL and ATR on Pleural Mesothelioma Cells. Mol Cancer Ther. 1;23(2):212-222. 2024. (PMID: 37802502)
- Jiang L, Zheng H, Ishida M, Lyu Q, Akatsuka S, Motooka Y, Sato K, Sekido Y, Nakamura K, Tanaka H, Ishikawa K, Kajiyama H, Mizuno M, Hori M, Toyokuni S. Elaborate cooperation of poly(rC)-binding proteins 1/2 and glutathione in ferroptosis induced by plasma-activated Ringer’s lactate. Free Radic Biol Med. 214:28-41. 2024. (PMID: 38325565)
2023
- Sekido Y, Sato T. NF2 alteration in mesothelioma. Front Toxicol. 25;5:1161995. 2023. (PMID: 37180489)
- Sato T, Akao K, Sato A, Tsujimura T, Mukai S, Sekido Y. Aberrant expression of NPPB through YAP1 and TAZ activation in mesothelioma with Hippo pathway gene alterations. Cancer Med. 12(12):13586-13598. 2023. (PMID: 37165917)
2022
- Wirawan A, Tajima K, Takahashi F, Mitsuishi Y, Winardi W, Hidayat M, Hayakawa D, Matsumoto N, Izumi K, Asao T, Ko R, Shimada N, Takamochi K, Suzuki K, Abe M, Hino O, Sekido Y, Takahashi K. A Novel Therapeutic Strategy Targeting the Mesenchymal Phenotype of Malignant Pleural Mesothelioma by Suppressing LSD1. Mol Cancer Res. 20(1):127-138. 2022. (PMID: 34593606)
- Yue L, Luo Y, Jiang L, Sekido Y, Toyokuni S. PCBP2 knockdown promotes ferroptosis in malignant mesothelioma. Pathol Int. 72(4):242-251. 2022. (PMID: 35089637)
- Nakashima K, Sakai Y, Hoshino H, Umeda Y, Kawashima H, Sekido Y, Ishizuka T, Kobayashi M. Sulfated Glycans Recognized by S1 Monoclonal Antibody can Serve as a Diagnostic Marker for Malignant Pleural Mesothelioma. Lung. 200(3):339-346. 2022. (PMID: 35394203)
- Fujibayashi E, Mukai S, Torigata K, Ando Y, Uchihashi T, Nozaki M, Tanaka S, Okada M, Kogo M, Nojima H, Yabuta N. LATS kinases and SLUG regulate the transition to advanced stage in aggressive oral cancer cells. Sci Rep. 20;12(1):12363. 2022. (PMID: 35859006)
- Hagiyama M, Mimae T, Wada A, Takeuchi F, Yoneshige A, Inoue T, Kotoku N, Hamada H, Sekido Y, Okada M, Ito A. Possible Therapeutic Utility of anti-Cell Adhesion Molecule 1 Antibodies for Malignant Pleural Mesothelioma. Front Cell Dev Biol. 12;10:945007. 2022. (PMID: 35903548)
- Suzuki K, Tange M, Yamagishi R, Hanada H, Mukai S, Sato T, Tanaka T, Akashi T, Kadomatsu K, Maeda T, Miida T, Takeuchi I, Murakami H, Sekido Y, Murakami-Tonami Y. SMG6 regulates DNA damage and cell survival in Hippo pathway kinase LATS2-inactivated malignant mesothelioma. Cell Death Discov. 5;8(1):446. 2022. (PMID:36335095)
2021
- Goto S, Sakoda Y, Adachi K, Sekido Y, Yano S, Eto M, Tamada K. Enhanced anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells in orthotopic and patient-derived xenograft tumor models. Cancer Immunol Immunother. 70(9):2503-2515. 202. (PMID: 33559069)
- Sato T, Mukai S, Ikeda H, Mishiro-Sato E, Akao K, Kobayashi T, Hino O, Shimono W, Shibagaki Y, Hattori S, Sekido Y. Silencing of SmgGDS, a Novel mTORC1 Inducer That Binds to RHEBs, Inhibits Malignant Mesothelioma Cell Proliferation. Mol Cancer Res. 19(5):921-931. 2021. (PMID: 33574130)
- Jiang L, Zheng H, Lyu Q, Hayashi S, Sato K, Sekido Y, Nakamura K, Tanaka H, Ishikawa K, Kajiyama H, Mizuno M, Hori M, Toyokuni S. Lysosomal nitric oxide determines transition from autophagy to ferroptosis after exposure to plasma-activated Ringer’s lactate. Redox Biol. 43:101989. 2021. (PMID: 33940548 )
- Kodama Y, Tanaka I, Sato T, Hori K, Gen S, Morise M, Matsubara D, Sato M, Sekido Y, Hashimoto N. Oxytocin receptor is a promising therapeutic target of malignant mesothelioma. Cancer Sci. 112(9):3520-3532. 2021. (PMID: 34115916 )
- Sato T, Nakanishi H, Akao K, Okuda M, Mukai S, Kiyono T, Sekido Y. Three newly established immortalized mesothelial cell lines exhibit morphological phenotypes corresponding to malignant mesothelioma epithelioid, intermediate, and sarcomatoid types, respectively. Cancer Cell Int. 18;21(1):546. 2021. (PMID: 34663305)
- Yamazaki S, Ohka F, Hirano M, Shiraki Y, Motomura K, Tanahashi K, Tsujiuchi T, Motomura A, Aoki K, Shinjo K, Murofushi Y, Kitano Y, Maeda S, Kato A, Shimizu H, Yamaguchi J, Adilijiang A, Wakabayashi T, Saito R, Enomoto A, Kondo Y, Natsume A. Newly established patient-derived organoid model of intracranial meningioma. Neuro Oncol. 2;23(11):1936-1948. 2021. (PMID: 34214169)
2020
- Kaneda A, Seike T, Danjo T, Nakajima T, Otsubo N, Yamaguchi D, Tsuji Y, Hamaguchi K, Yasunaga M, Nishiya Y, Suzuki M, Saito JI, Yatsunami R, Nakamura S, Sekido Y, Mori K. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Res. 1;10(12):4399-4415. 2020. (PMID: 33415007)
2019
- Matsushita A, Sato T, Mukai S, Fujishita T, Mishiro-Sato E, Okuda M, Aoki M, Hasegawa Y, Sekido Y. TAZ activation by Hippo pathway dysregulation induces cytokine gene expression and promotes mesothelial cell transformation. Oncogene 38:1966-1978, 2019. (PMID: 30401981)
2018
- McCambridge AJ, Napolitano A, Mansfield AS, Fennell DA, Sekido Y, Nowak AK, Reungwetwattana T, Mao W, Pass HI, Carbone M, Yang H, Peikert T: State of the Art: Advances in Malignant Pleural Mesothelioma in 2017. J Thorac Oncol. pii: S1556-0864(18)30175-8, 2018. (PMID: 29524617)
- Sekido Y: Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers (Basel). 10(4). pii: E90, 2018. (PMID: 29565815)
- Sato T, Sekido Y: NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma. Int J Mol Sci. 19(4). pii: E988, 2018. (PMID: 29587439)
- Tanaka I, Sato M, Kato T, Goto D, Kakumu T, Miyazawa A, Yogo N, Hase T, Morise M, Sekido Y, Girard L, Minna JD, Byers LA, Heymach JV, Coombes KR, Kondo M, Hasegawa Y. eIF2β, a subunit of translation-initiation factor EIF2, is a potential therapeutic target for non-small cell lung cancer. Cancer Sci. 109:1843-1852, 2018. (PMID: 29624814)
- Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman DI, Tarpey P, Danilova L, Drill E, Gibb EA, Bowlby R, Kanchi R, Osmanbeyoglu HU, Sekido Y, Takeshita J, Newton Y, Graim K, Gupta M, Gay CM, Diao L, Gibbs DL, Thorsson V, Iype L, Kantheti HS, Severson DT, Ravegnini G, Desmeules P, Jungbluth AA, Travis WD, Dacic S, Chirieac LR, Galateau-Salle F, Fujimoto J, Husain AN, Silveira HC, Rusch VW, Rintoul RC, Pass H, Kindler H, Zauderer MG, Kwiatkowski DJ, Bueno R, Tsao AS, Creaney J, Lichtenberg T, Leraas K, Bowen J, Research Network T, Felau I, Zenklusen JC, Akbani R, Cherniack AD, Byers LA, Noble MS, Fletcher JA, Robertson G, Shen R, Aburatani H, Robinson BW, Campbell P, Ladanyi M. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. pii: CD-18-0804, 2018. (PMID: 30322867)
- Ohara Y, Chew SH, Misawa N, Wang S, Somiya D, Nakamura K, Kajiyama H, Kikkawa F, Tsuyuki Y, Jiang L, Yamashita K, Sekido Y, Lipson KE, Toyokuni S. Connective tissue growth factor-specific monoclonal antibody inhibits growth of malignant mesothelioma in an orthotopic mouse model. Oncotarget. 9:18494-18509, 2018. (PMID: 29719620)
2017
- Tanaka K, Osada H, Murakami-Tonami Y, Horio Y, Hida T, Sekido Y: Statin suppresses Hippo pathway-inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett 385: 215-224, 2017. (PMID: 27773750)
- Kakumu T, Sato M, Goto D, Kato T, Yogo N, Hase T, Morise M, Fukui T, Yokoi K, Sekido Y, Girard L, Minna JD, Byers LA, Heymach JV, Coombes KR, Kondo M, Hasegawa Y: Identification of Proteasomal Catalytic Subunit PSMA6 as a Therapeutic Target for Lung Cancer. Cancer Sci 108(4):732-743, 2017. (PMID: 28165654)
- Fukushima K, Wang M, Naito Y, Uchihashi T, Kato Y, Mukai S, Yabuta N, Nojima H: GAK is phosphorylated by c-Src and translocated from the centrosome to chromatin at the end of telophase. Cell Cycle 31:1-13, 2017. (PMID: 28135906)
- Kato T, Sato T, Yokoi K, Sekido Y: E-cadherin expression is correlated with focal adhesion kinase inhibitor resistance in Merlin-negative malignant. Oncogene 36(39):5522-5531, 2017. (PMID: 28553954)
- Chew SH, Okazaki Y, Akatsuka S, Wang S, Jiang L, Ohara Y, Ito F, Saya H, Sekido Y, Toyokuni S: Rheostatic CD44 isoform expression and its association with oxidative stress in human malignant mesothelioma. Free Radic Biol Med 106:91-99, 2017. (PMID: 28185919)
- Kakumu T, Sato M, Goto D, Kato T, Yog, N, Hase T, Morise M, Fukui T, Yokoi K, Sekido Y, Girard L, Minna JD, Byers LA, Heymach JV, Coombes KR, Kondo M, Hasegawa Y: Identification of proteasomal catalytic subunit PSMA6 as a therapeutic target for lung cancer. Cancer Sci 108(4):732-743, 2017. (PMID: 28165654)
- Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL: Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat Cell Biol 19(8):996-1002,2017. (PMID: 28752853)
- Shishido Y, Tomoike F, Kimura Y, Kuwata K, Yano T, Fukui K, Fujikawa H, Sekido Y, Murakami-Tonami Y, Kameda T, Shuto S, Abe H: A Covalent G-site Inhibitor for Glutathione S-Transferase Pi (GSTP1-1). Chem Commun (Camb) 53(81):11138-11141,2017. (PMID: 28848941)
- Sato T, Higuchi Y, Shibagaki Y, Hattori S: Phosphoproteomic Analysis Identifies Signaling Pathways Regulated by Curcumin in Human Colon Cancer Cells. Anticancer Res 37(9):4789-4798, 2017. (PMID: 28870897)
- Shigeeda W, Shibazaki M, Yasuhira S, Kaneko Y, Masuda T, Tanita T, Sato T, Sekido Y, Maesawa C: Hyaluronic acid enhances cell migration and invasion via the YAP1/TAZ-RHAMM axis in malignant pleural mesothelioma. Oncotarget 8(55):93729-93740, 2017. (PMID: 29212185)
2016
- Wang S, Jiang L, Han Y, Hwu Chew S, Ohara Y, Akatsuka S, Weng L, Kawaguchi K, Fukui T, Sekido Y, Yokoi K, Toyokuni S: Urokinase-type plasminogen activator receptor promotes proliferation and invasion with reduced cisplatin sensitivity in malignant mesothelioma. Oncotarget 7: 69565-69578, 2016. (PMID: 27602956)
- Ito T, Matsubara D, Tanaka I, Makiya K, Tanei ZI, Kumagai Y, Shiu SJ, Nakaoka HJ, Ishikawa S, Isagawa T, Morikawa T, Shinozaki-Ushiku A, Goto Y, Nakano T, Tsuchiya T, Tsubochi H, Komura D, Aburatani H, Dobashi Y, Nakajima J, Endo S, Fukayama M, Sekido Y, Niki T, Murakami, Y: Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci 107: 1527-1538, 2016. (PMID: 27418196)
- Murakami-Tonami Y, Ikeda H, Yamagishi R, Inayoshi M, Inagaki S, Kishida S, Komata Y, Jan Koster, Takeuchi I, Kondo Y, Maeda T, Sekido Y, Murakami H, Kadomatsu K: SGO1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells. Sci Rep 19;6:31615, 2016. (PMID: 27539729)
- Yabuta N, Yoshida K, Mukai S, Kato Y, Torigata K, Nojima H: Large tumor suppressors 1 and 2 regulate Aurora-B through phosphorylation of INCENP to ensure completion of cytokinesis. Heliyon 20;2, 2016. (PMID: 27512725)
- Torigata K, Okuzaki D, Mukai S, Hatanaka A, Ohka F, Motooka D, Nakamura S, Ohkawa Y, Yabuta N, Kondo Y, Nojima H : LATS2 Positively Regulates Polycomb Repressive Complex. PLoS One 19:11, 2016. (PMID: 27434182)
- Mizuuchi H, Suda K, Murakami I, Sakai K, Sato K, Kobayashi Y, Shimoji M, Chiba M, Sesumi Y, Tomizawa K, Takemoto T, Sekido Y, Nishio K, Mitsudomi T: Oncogene swap as a novel mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor in lung cancer. Cancer Sci 107: 461-8, 2016. (PMID: 26845230)
- Hikasa H, Sekido Y, Suzuki A: Merlin/NF2-Lin28B-let-7 Is a Tumor-Suppressive Pathway that Is Cell-Density Dependent and Hippo Independent. Cell Rep 14: 2950-61, 2016. (PMID: 26997273)
- Nishio M, Sugimachi K, Goto H, Wang J, Morikawa T, Miyachi Y, Takano Y, Hikasa H, Itoh T, Suzuki SO, Kurihara H, Aishima S, Leask A, Sasaki T, Nakano T, Nishina H, Nishikawa Y, Sekido Y, Nakao K, Shin-Ya K, Mimori K, Suzuki A: Dysregulated YAP1/TAZ and TGF-β signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci U S A 113: E71-80, 2016. (PMID: 26699479)
2015
- Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, Horio Y, Hata A, Kaji R, Fujita S, Sekido Y, Kodaira T, Kokubo M, Katakami N, Yatabe Y: EGFR Mutation Impact on Definitive Concurrent Chemoradiation Therapy for Inoperable Stage III Adenocarcinoma. J Thorac Oncol 10: 1720-5, 2015. (PMID: 26743855)
- Hakiri S, Osada H, Ishiguro F, Murakami H, Murakami-Tonami Y, Yokoi K, Sekido Y: Functional differences between wild-type and mutant-type BRCA1-associated protein 1 tumor suppressor against malignant mesothelioma cells. Cancer Sci 106: 990-9, 2015. (PMID: 26011428)
- Tanahashi K, Natsume A, Ohka F, Motomura K, Alim A, Tanaka I, Senga T, Harada I, Fukuyama R, Sumiyoshi N, Sekido Y, Wakabayashi T: Activation of Yes-Associated Protein in Low-Grade Meningiomas Is Regulated by Merlin, Cell Density, and Extracellular Matrix Stiffness. J Neuropathol Exp Neurol 74: 704-9, 2015. (PMID: 26049897)
- Mizuuchi H, Suda K, Sato K, Tomida S, Fujita Y, Kobayashi Y, Maehara Y, Sekido Y, Nishio K, Mitsudomi T: Collateral Chemoresistance to Anti- Microtubule Agents in a Lung Cancer Cell Line with Acquired Resistance to Erlotinib. PLoS ONE 10: e0123901, 2015. (PMID: 25875914)
- Nakaguro M, Kiyonari S, Kishida S, Cao D, Murakami-Tonami Y, Ichikawa H, Takeuchi I, Nakamura S, Kadomatsu K: Nucleolar protein PES1 is a marker of neuroblastoma outcome and is associated with neuroblastoma differentiation. Cancer Sci 106: 237-43, 2015. (PMID: 25557119)
- Yamashita R, Sato M, Kakumu T, Hase T, Yogo N, Maruyama E, Sekido Y, Kondo M, Hasegawa Y: Growth inhibitory effects of miR-221 and miR-222 in non-small cell lung cancer cells. Cancer Med 4: 551-64, 2015. (PMID: 25641933)
- Gao W, Gu Y, Li Z, Cai H, Peng Q, Tu M, Kondo Y, Shinjo K, Zhu Y, Zhang J, Sekido Y, Han B, Qian Z, Miao Y: miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene 34: 1629-40, 2014. (PMID: 24769899)
- Li Q, Wang W, Machino Y, Yamada T, Kita K, Oshima M, Sekido Y, Tsuchiya M, Suzuki Y, Nan-Ya K, Iida S, Nakamura K, Iwakiri S, Itoi K, Yano S: Therapeutic activity of glycoengineered anti-GM2 antibodies against malignant pleural mesothelioma. Cancer Sci 106: 102-7, 2015. (PMID: 25421609)
- Tanaka I, Osada H, Fujii M, Fukatsu A, Hida T, Horio Y, Kondo Y, Sato A, Hasegawa Y, Tsujimura T, Sekido Y: LIM-domain protein AJUBA suppresses malignant mesothelioma cell proliferation via Hippo signaling cascade. Oncogene 34:73-83, 2015. (PMID: 24336325)
2014
- Suda K, Mizuuchi H, Murakami I, Uramoto H, Tanaka F, Sato K, Takemoto T, Iwasaki T, Sekido Y, Yatabe Y, Mitsudomi T: CRKL amplification is rare as a mechanism for acquired resistance to kinase inhibitors in lung cancers with epidermal growth factor receptor mutation. Lung Cancer 85: 147-51, 2014. (PMID: 24939008)
- Fernandez-Cuesta L, Plenker D, Osada H, Sun R, Menon R, Leenders F, Ortiz-Cuaran S, Peifer M, Bos M, DaBler J, Malchers F, Schottle J, Vogel W, Dahmen I, Koker M, Ullrich RT, Wright GM, Russell PA, Wainer Z, Solomon B, Brambilla E, Nagy-Mignotte H, Moro-Sibilot D, Brambilla CG, Lantuejoul S, Altmuller J, Becker C, Nurnberg P, Heuckmann JM, Stoelben E, Petersen I, Clement JH, Sanger J, Muscarella LA, la Torre A, Fazio VM, Lahortiga I, Perera T, Ogata S, Parade M, Brehmer D, Vingron M, Heukamp LC, Buettner R, Zander T, Wolf J, Perner S, Ansen S, Haas SA, Yatabe Y, Thomas RK: CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discovery 4: 415-22, 2014. (PMID: 24469108)
- Murakami-Tonami Y, Kishida S, Takeuchi I, Katou Y, Maris JM, Ichikawa H, Kondo Y, Sekido Y, Shirahige K, Murakami H, Kadomatsu K: Inactivation of SMC2 shows a synergistic lethal response in MYCN-amplified neuroblastoma cells. Cell Cycle 13: 1115-31, 2014. (PMID: 24553121)
- Okamoto Y, Shinjo K, Shimizu Y, Sano T, Yamao K, Gao W, Fujii M, Osada H, Sekido Y, Murakami S, Tanaka Y, Joh T, Sato S, Takahashi S, Wakita T, Zhu J, Issa JP, Kondo Y: Hepatitis virus infection affects DNA methylation in mice with humanized livers. Gastroenterology 146: 562-72, 2014. (PMID: 24184133)
- Fukatsu A, Ishiguro F, Tanaka I, Kudo T, Nakagawa K, Shinjo K, Kondo Y, Fujii M, Hasegawa Y, Tomizawa K, Mitsudomi T, Osada H, Hata Y, Sekido Y: RASSF3 downregulation increases malignant phenotypes of non-small cell lung cancer. Lung Cancer 83: 23-9, 2014. (PMID: 24246507)
- Chew SH, Okazaki Y, Nagai H, Misawa N, Akatsuka S, Yamashita K, Jiang L, Yamashita Y, Noguchi M, Hosoda K, Sekido Y, Takahashi T, Toyokuni S: Cancer-promoting role of adipocytes in asbestos-induced mesothelial carcinogenesis through dysregulated adipocytokine production. Carcinogenesis 35: 164-72, 2014. (PMID: 23917077)
2013
- Yamada T, Takeuchi S, Fujita N, Nakamura A, Wang W, Li Q, Oda M, Mitsudomi T, Yatabe Y, Sekido Y, Yoshida J, Higashiyama M, Noguchi M, Uehara H, Nishioka Y, Sone S, Yano S: Akt kinase-interacting protein1, a novel therapeutic target for lung cancer with EGFR-activating and gatekeeper mutations. Oncogene 32: 4427-35, 2013. (PMID: 23045273)
- Natsume A, Ito M, Katsushima K, Ohka F, Hatanaka A, Shinjo K, Sato S, Takahashi S, Ishikawa Y, Takeuchi I, Shimogawa H, Uesugi M, Okano H, Kim SU, Wakabayashi T, Issa JP, Sekido Y, Kondo Y: Chromatin regulator PRC2 is a key regulator of epigenetic plasticity in glioblastoma. Cancer Res 73: 4559-70, 2013. (PMID: 23720055)
- Sekido Y: Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 34: 1413-9, 2013. (PMID: 23677068)
- Xue X, Gao W, Sun B, Xu Y, Han B, Wang F, Zhang Y, Sun J, Wei J, Lu Z, Zhu Y, Sato Y, Sekido Y, Miao Y, Kondo Y: Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 32: 1724-34, 2013. (PMID: 22614011)
- Nakagawa T, Takeuchi S, Yamada T, Ebi H, Sano T, Nanjo S, Ishikawa D, Sato M, Hasegawa Y, Sekido Y, Yano S: EGFR-TKI resistance due to BIM polymorphism can be circumvented by in combination with HDAC inhibition. Cancer Res 73: 2428-34, 2013. (PMID: 23382048)
- Abe S, Morita Y, Kato-Kaneko M, Hanibuchi M, Tsujimoto Y, Goto H, Kakiuchi S, Aono Y, Huang J, Sato S, Kishuku M, Taniguchi Y, Azuma M, Kawazoe K, Sekido Y, Yano S, Akiyama S, Sone S, Minakuchi K, Kato Y, Nishioka Y: A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. J Immunol 190: 6239-40, 2013. (PMID: 23690472)
- Satoh M, Takemura Y, Hamada H, Sekido Y, Kubota S: EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int 13:19, 2013. (PMID: 23432995)
- Ishiguro F, Murakami H, Mizuno T, Fujii M, Kondo Y, Usami N, Taniguchi T, Yokoi K, Osada H, Sekido Y: Membranous expression of activated leukocyte cell adhesion molecule (ALCAM) contributes to poor prognosis and malignant phenotypes of non-small cell lung cancer. J Surg Research 179: 24-32, 2013. (PMID: 22985775)

