Division of Immune Response
Introduction
Overview
We aim to investigate and elucidate the detailed mechanisms by which immune cells recognize and attack cancer cells (cancer immunity). We also aim to develop novel cancer immunotherapies and therapeutic drugs which strengthen cancer immunity.

In the Division of Immune Response, we focus on the cancer immunity of immune cells called natural killer (NK) cells. Research on cancer immunity and development of cancer immunotherapy are mainly focused on immune cells called T cells. However, the therapeutic efficacy of current cancer immunotherapy is not sufficient and only a limited number of cancer types can be cured. Thus, it is necessary to develop new cancer immunotherapy that strengthens the function of immune cells involved in cancer immunity other than T cells. NK cells have the ability of attacking and eliminating cancer cells that T cells cannot eliminate so that they would be useful for the development of next-generation cancer immunotherapy. However, no established treatment currently promotes the cancer immunity of NK cells. We would like to contribute to the eradication of cancer through research on NK cells. To achieve this, we first aim to understand NK cells more deeply by elucidating the series of phenomena in which NK cells encounter cancer cells, perceive them as something to be eliminated (recognition), become able to attack cancer (activation), attack them (killing, called cytotoxicity), change their properties (differentiation), and die (cell death). Furthermore, by utilizing the knowledge gained through basic research, we aim to develop cancer immunotherapy that can cure cancer using NK cells through research and development of drugs that enhance cancer immunity of NK cells and through translational research into cancer immunotherapy using genetically modified NK cells.
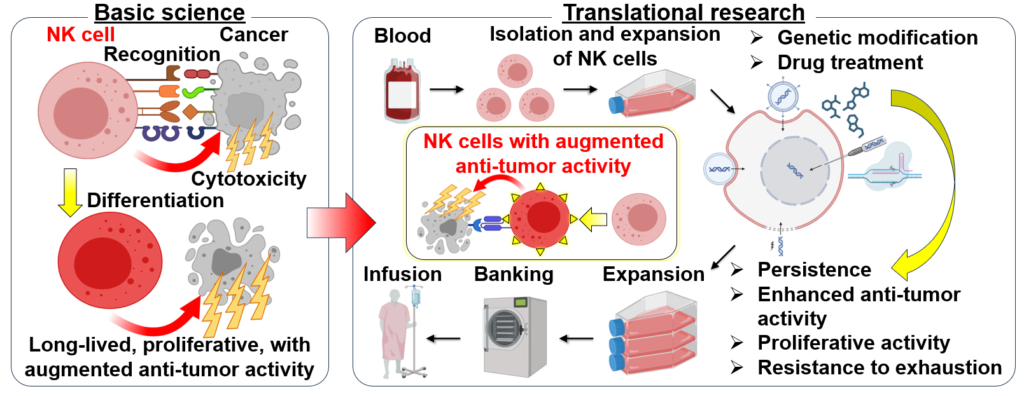
Aim
We aim to develop cancer immunotherapy that can cure cancer by using NK cells. To achieve this goal, we dedicate ourselves to not only basic research to understand the nature of cancer immunity by NK cells, but also translational research to artificially enhance the cancer immunity of NK cells.
Contact
Chief of Division of Immune Response
Tsukasa Nabekura
1-1 Kanokoden, Chikusa Ward, Nagoya, 464-8681
Tel: 052-762-6111 (ext.) 7020
E-mail: t.nabekura@aichi-cc.jp
For the public
Overview of research activities
In our body NK cells and T cells are present as immune cells that eliminate cancer. By elucidating the mechanisms by which these immune cells recognize cancer cells, undergo activation, and then kill cancer cells (as known as cancer immunity), we aim to strengthen cancer immunity and develop more effective treatments and drugs for cancer. In Division of Immune Response, we are focusing on cancer immunity, particularly of NK cells, among the immune cells that are responsible for cancer immunity. Current research and development of cancer immunotherapy is centered on T cells. However, the therapeutic efficacy of current cancer immunotherapy is not sufficient, and only a limited number of cancer types can be cured. Additionally, current immunotherapy sometimes causes severe adverse effects. Therefore, it is essential to develop safer and more effective cancer immunotherapies. NK cells have the ability of attacking and eliminating cancer cells that T cells cannot eliminate, so NK cells would be useful for the development of next-generation cancer immunotherapy. However, to date, neither drugs nor immunotherapy that enhance the function of NK cells have been established. Our ultimate goal is to contribute to the eradication of cancer through research on NK cells. Therefore, we first dedicate ourselves to basic research to elucidate and gain a deeper understanding of the molecular mechanisms by which NK cells recognize and attack cancer cells. Furthermore, with the knowledge gained through basic research, we aim to translate the achievement into translational research, such as, research and development of cancer immunotherapies and drugs that enhance cancer immunity of NK cells. Eventually, we will confirm the safety and efficacy of new treatments through clinical trials, and ultimately aim to provide a treatment that uses NK cells to cure cancer.
Our research
(1) What are NK cells?
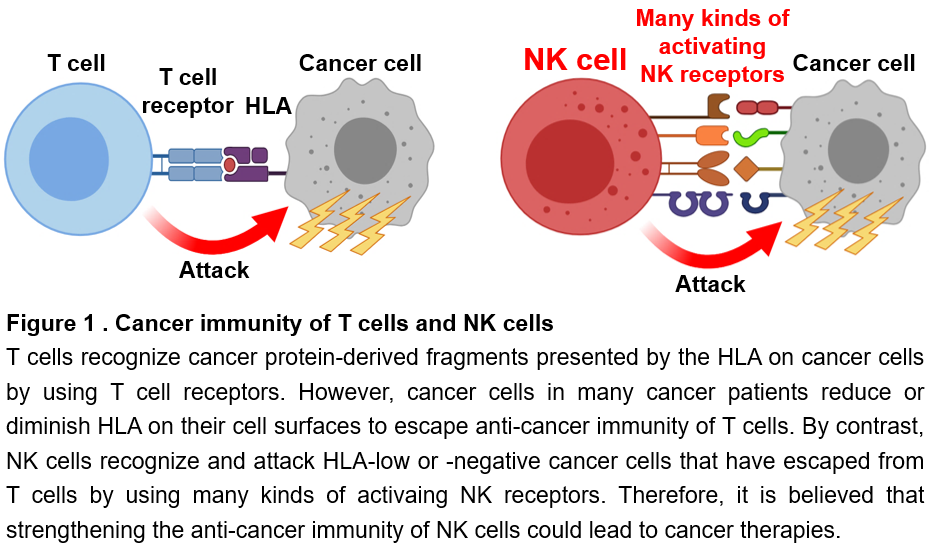
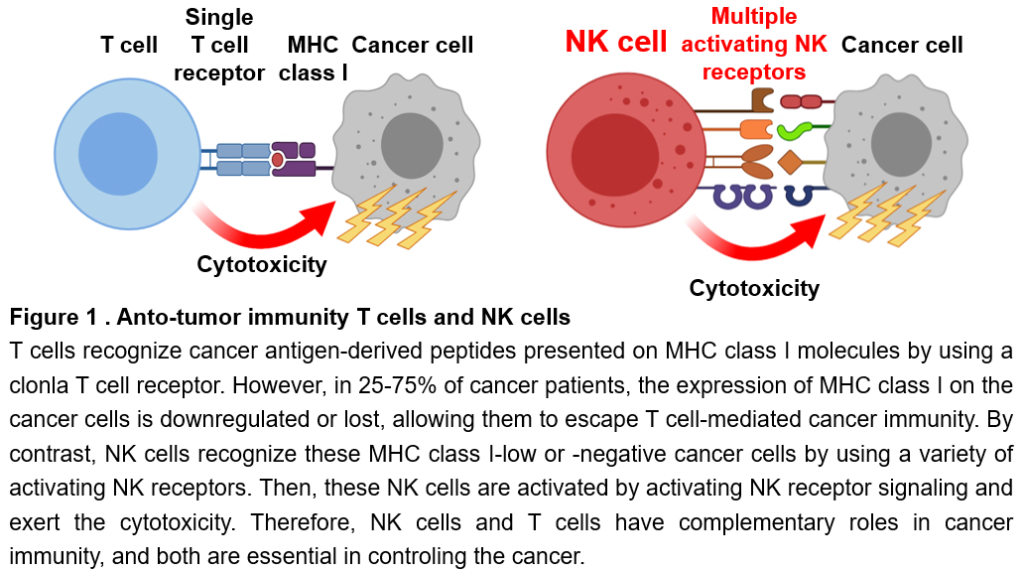
NK cells and T cells are present as immune cells that eliminate cancer. T cells recognize cancer-specific protein-derived fragments presented on human leukocyte antigens (HLA) on the surface of cancer cells with a protein called a T cell receptor. T cells are then activated and attack the cancer cells (Figure 1). However, it is well known that cancer cells reduce or lose HLA on their cell surface as they become malignant, making them less recognizable by T cells. Finally, cancer cells escape the cancer immunity of T cells. In fact, in 25 to 75% of cancer patients, HLA is reduced or lost on cancer cells. On the other hand, NK cells can recognize, become activated, and attack cancer cells with reduced HLA that T cells cannot recognize. NK cells recognize them using various types of proteins called NK receptors (Figure 1). Thus, NK cells and T cells play complementary roles in cancer immunity. Therefore, if the cancer immunity of NK cells can be enhanced, it may be possible to treat cancers that cannot be cured by current cancer immunotherapy using T cells. Nowadays, NK cells are attractive tool as a trump card in cancer treatment. However, to date, neither cancer immunotherapies nor therapeutic drugs that enhance cancer immunity of NK cells have been developed.
We are conducting basic research to elucidate the molecular mechanisms underlying a series of phenomena by which NK cells recognize, are activated, and attack cancer cells. Furthermore, by utilizing the knowledge gained from basic research, we are conducting translational research to develop genetic modifications and drugs that enable NK cells to recognize and attack cancer cells more efficiently, as well as cancer immunotherapy using NK cells with enhanced functions.
(2) Research topics of our division
Since both NK cells and T cells play essential roles in cancer immunity, a therapy that enhances the cancer immunity of NK cells may have a synergistic therapeutic effect when used in combination with a conventional therapy that enhances the cancer immunity of T cells. In order to enhance NK cell-mediated cancer immunity, we are taking a wide range of approaches, from basic research on NK cells to translational research.
1. Identification of NK cell receptors, ligands, and immune checkpoints, and research into drug discovery targeting these
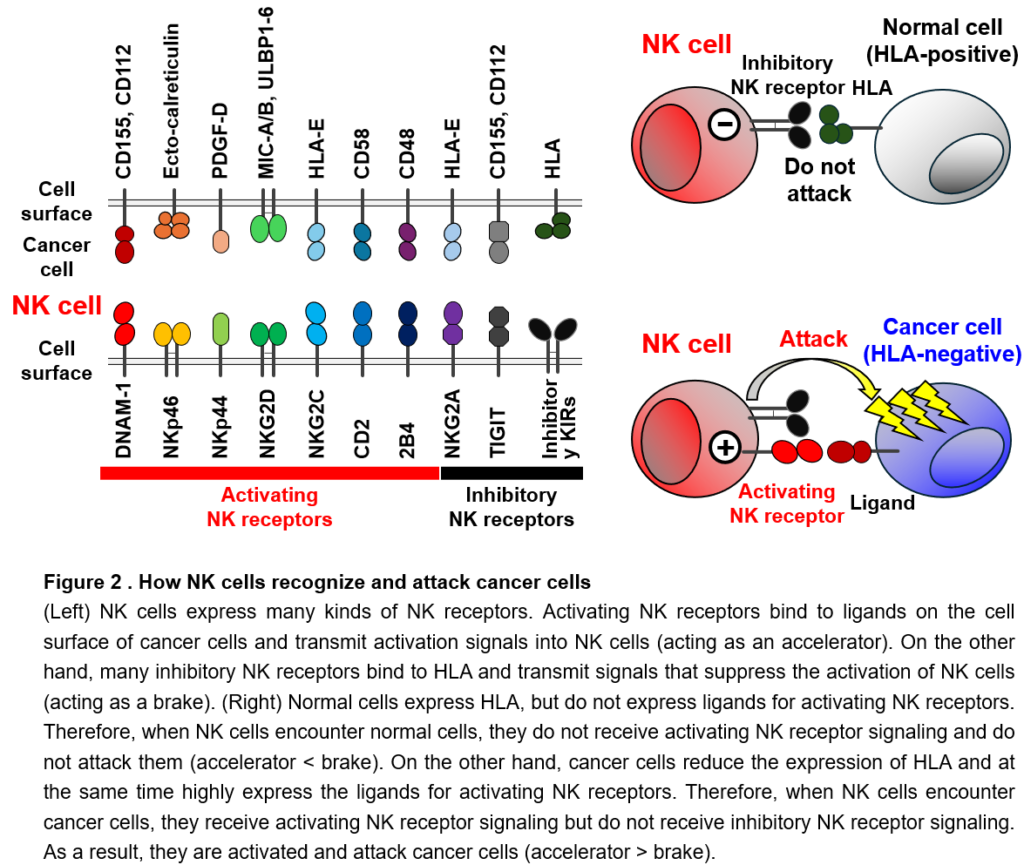
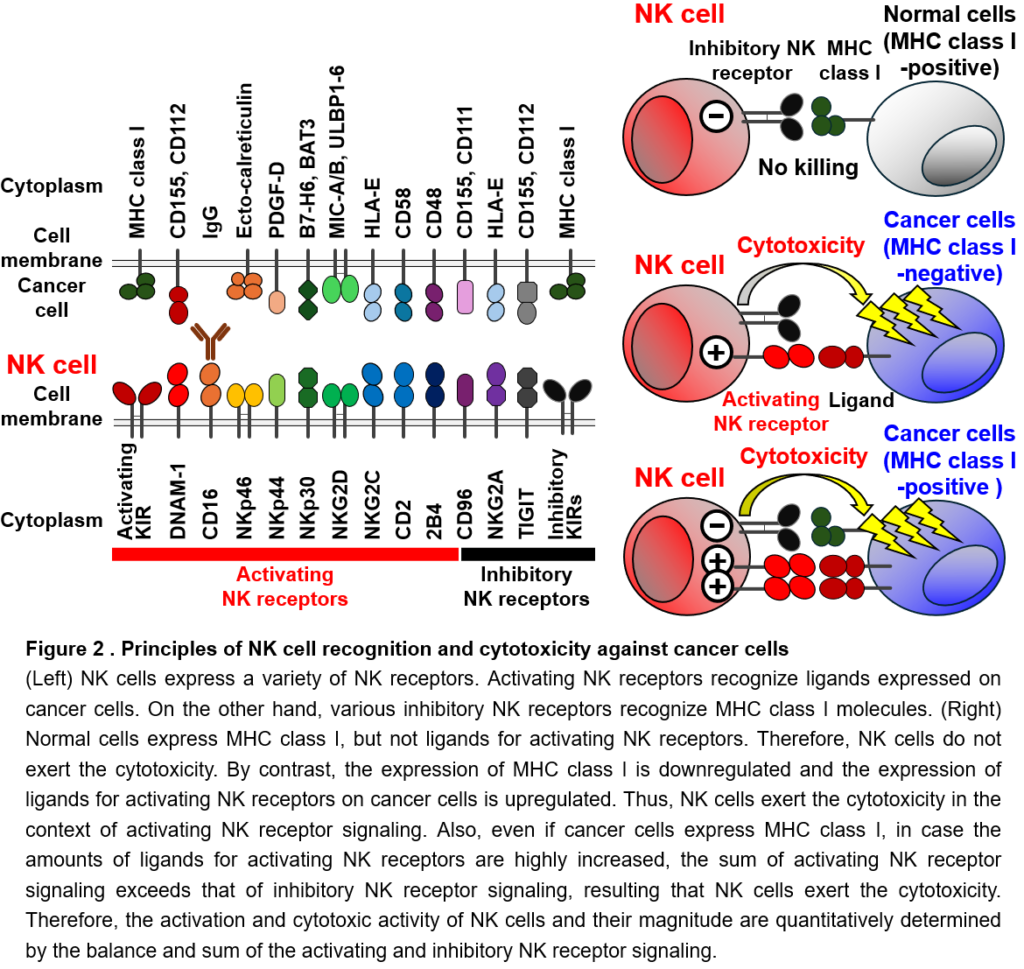
NK cells recognize cancer cells using expressing many types of NK receptors. Individual NK receptors specifically bind to different proteins (called ligands) expressed on the surface of cancer cells (Figure 2). There are two types of NK receptors: activating NK receptors, which transmit signals that activate NK cells, and inhibitory NK receptors, which transmit signals that suppress the activation of NK cells. Activating NK receptors bind to ligands that are abundantly expressed on the surface of cancer cells and induces the activation of NK cells (acting as an accelerator). On the other hand, many inhibitory NK receptors bind to HLA and suppress the activation and attack of NK cells (acting as a brake). Normal cells do not express ligands for activating NK receptors and sufficiently express HLA, which is the ligand for inhibitory NK receptors. Thus, NK cells do not receive activating NK receptor signals when they encounter normal cells, so they are not activated and do not attack normal cells (accelerator < brake). On the other hand, cancer cells highly express ligands for activating NK receptors and also reduce the expression of HLA to escape from T cells. Thus, when NK cells encounter cancer cells, they receive activating NK receptor signals, but do not receive inhibitory signals through inhibitory NK receptors. As a result, NK cells are activated and attack cancer cells (accelerator > brake). In this way, NK cells attack only cancer cells, but not normal cells, depending on the balance of signals they receive through activating NK receptors (accelerator) and inhibitory NK receptors (brake). The magnitude of activation and killing activity of NK cells is also controlled by the strength of the signal from the activating NK receptor (Figure 2). However, all NK receptors for ligands highly expressed on cancer cells have not been identified yet. In addition, all these ligands on cancer cells have not been identified yet. We try to identify and functionally analyze these unknown NK receptors and their ligands to elucidate their roles in cancer immunity and to link them to clinical applications by using them to artificially enhance NK cell activation.
In addition, since there are a limited number of previous studies on receptors and signaling molecules that suppress the activation of NK cells, in particular, we are focusing on molecules that suppress the cancer immunity of NK cells. Immune checkpoint molecules suppress the activation and function of T cells. Immune checkpoint inhibitors, which have hot topics in recent years, are a modality that enhances the cancer immunity of T cells by inhibiting immune checkpoint molecules. Immune checkpoint inhibitors promote the activation of cancer-specific T cells. On the other hand, NK cells scarcely express any immune checkpoint molecules that have been discovered so far. Therefore, a strategy for enhancing the cancer immunity of NK cells is to newly discover immune checkpoint molecules of NK cells and develop drugs that inhibit this function. Currently, we have identified an immune checkpoint molecule of NK cells and are analyzing the function. If we can inhibit the function of immune checkpoint molecules of NK cells by genetic modification or drugs, it would be expected that NK cells can be able to attack cancer cells more efficiently. Currently, we are working to elucidate the molecular mechanism that suppresses the cancer immunity of NK cells, and at the same time, we are searching for compounds and approved drugs that enhance the cancer immunity of NK cells.
2. Differentiation of memory NK cells
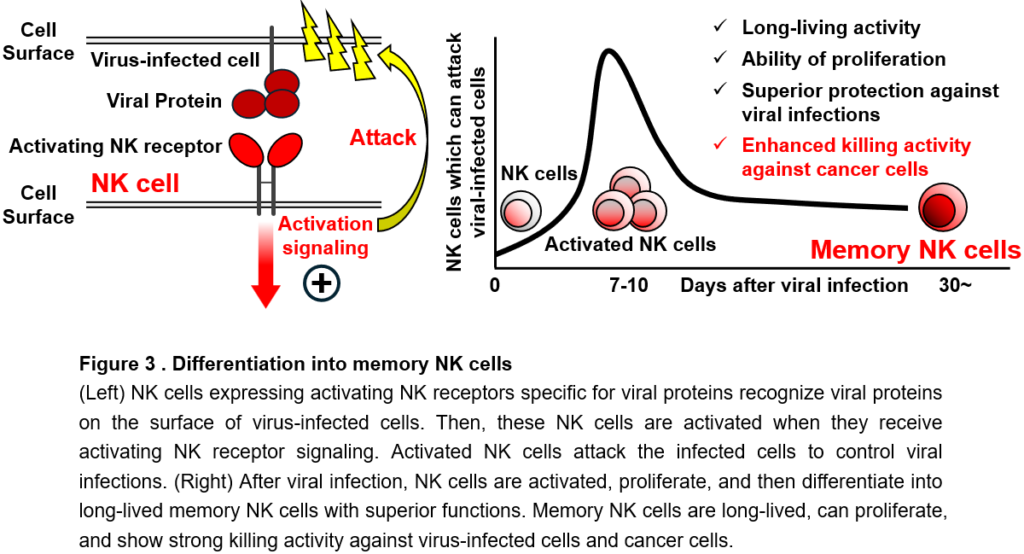
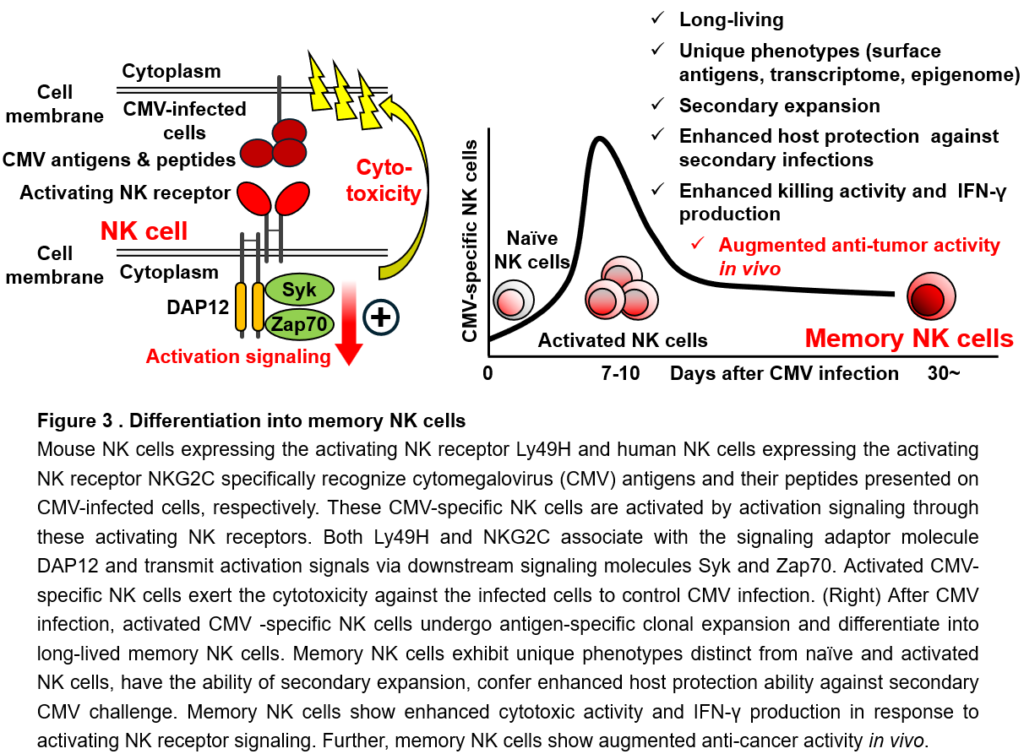
The greatest feature of the immune system is immunological memory. Immunological memory is a phenomenon in which, when a pathogen that has previously infected the body invades again, a faster and stronger immune response is triggered and also superior host defense against the same pathogen is maintained for a long time. Vaccines for infectious diseases are a medical application of immunological memory. Immunological memory is achieved by the differentiation (irreversible change in functional properties) of T cells and B cells into cells called memory T cells and memory B cells, respectively. These memory cells are long-lived cells that survives for several years or even a lifetime and show enhanced functions, such as strong killing activity against pathogens or cancer cells that they have encountered in the past, when they encounter them again. In recent years, it has been reported that memory T cells specific for cancer are important for sustained and effective cancer immunity. On the other hand, it has been reported that the longevity of NK cells seems up to 1 to 2 weeks in the body and die after attacking cancer cells. Therefore, since their discovery, it has been believed that NK cells do not differentiate into long-lived cells like memory T cells and are not involved in immunological memory. However, in recent years, a discovery that overturns the conventional theory has been made: after viral infections, NK cells proliferate in response to activation signals through activated NK receptors and then differentiate into a long-lived memory NK cells with enhanced killing activity, contributing to long-term host defense against viral infections. Unlike unsensitized (as known as naïve) NK cells, memory NK cells have a long life span, can proliferate again in response to secondary infections that have infected in the past, and can efficiently attack and eliminate these virus-infected cells (Figure 3). We have discovered that memory NK cells can exert strong killing activity against cancer cells (Figure 3). Therefore, memory NK cells would be useful for the development of new cancer immunotherapy. However, the mechanisms by which NK cells not only differentiate into memory NK cells but also obtain and maintain enhanced functions have not been fully elucidated. In addition, unlike viral infection, the differentiation of memory NK cells has not been observed in cancer patients, suggesting that some conditions for NK cells to differentiate into memory NK cells are not met in cancer.
We aim to apply the enhanced cellular functions of memory NK cells to cancer treatment. As a first step, we are conducting basic research to elucidate the molecular mechanisms that control the differentiation and function of memory NK cells. Furthermore, in order to apply the results of this basic research to cancer immunity, we are working to establish a method to artificially differentiate NK cells into memory NK cells and develop next-generation NK cell cancer immunotherapy that utilizes the strong cancer immunity of memory NK cells.
3. Development of new NK cell immunotherapy
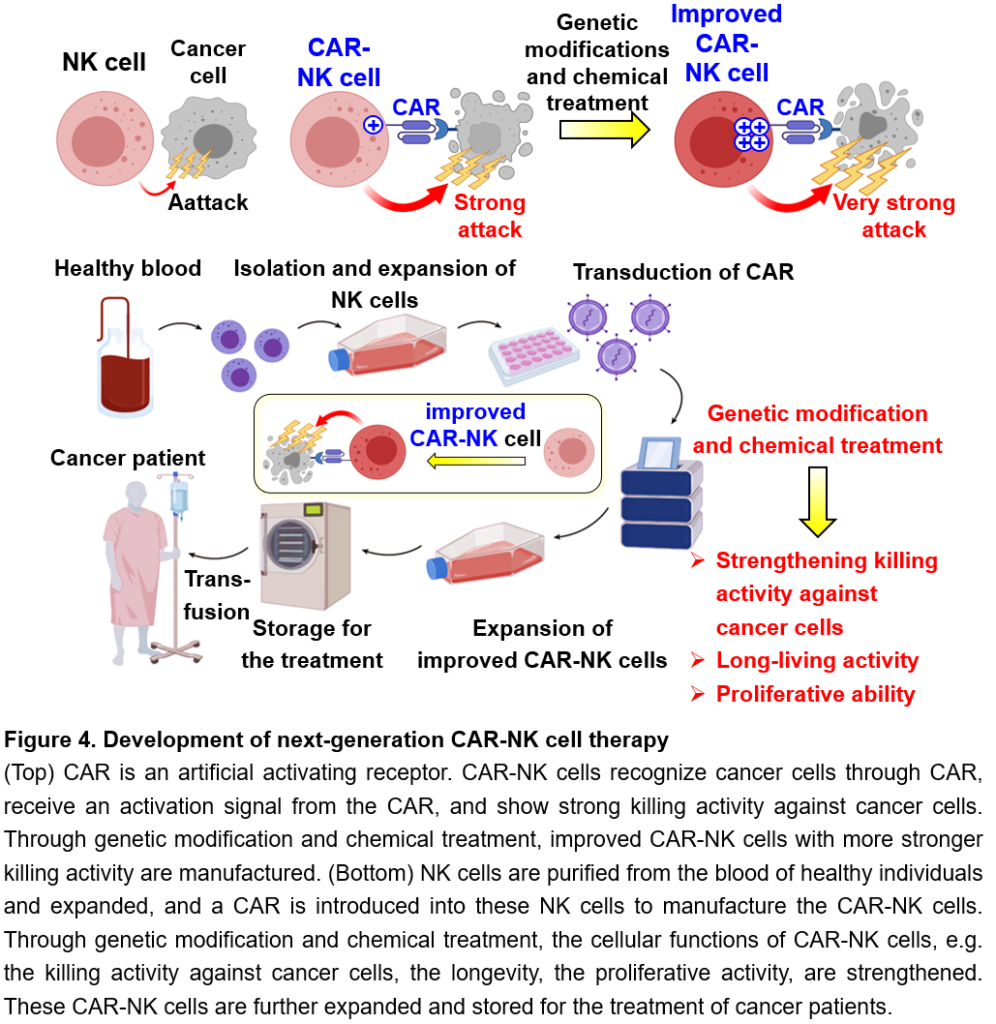
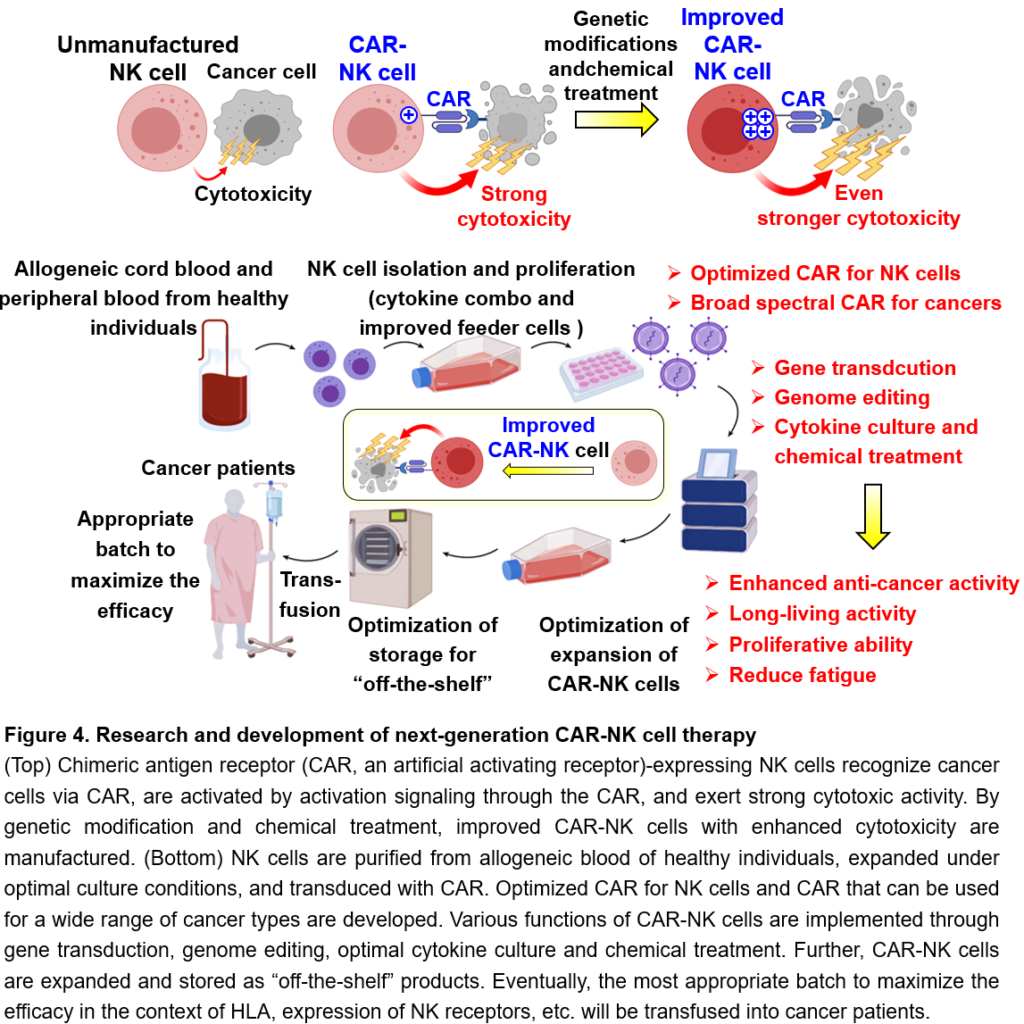
In recent years, immune cell therapy for cancer, which uses genetically modified immune cells as a therapeutic agent, has been attracting attention. In particular, CAR (Chimeric Antigen Receptor, or CAR) T cell therapy, in which cancer patients’ T cells are genetically modified to express an artificial activating receptor that can recognize cancer cells and then infused into the patient, has shown high therapeutic efficacy against several types of blood cancers (leukemia and lymphoma). CAR binds to a protein that is highly expressed in cancer cells and transmits a strong activation signal to T cells, enabling the T cells to attack cancer cells strongly. However, CAR T cell therapy often causes serious, sometimes fatal, side effects. In addition, since CAR T cells need to be individually manufactured using T cells in the blood from the patients, there is a risk of missing the treatment opportunity in case of rapidly progressing cancers, because the manufacturing of CAR T cells takes time or fails in a certain percentage of cases. In order to overcome these challenges of CAR T cells, CAR-NK cell therapy has been developed. The latest clinical trial of CAR-NK cell therapy has confirmed its efficacy against blood cancers without any serious side effects. Technically, CAR-NK cells can be manufactured from the blood of healthy individuals or umbilical cord blood and then can be expanded in large quantities after preparation, which has the great advantage that these CAR-NK cells can be stored as “off-the-shelf” drugs for the treatment for cancer patients. However, current CAR-NK cells have a short life span in the patient’s body after transfusion and do not expand even when they encounter cancer cells, resulting in the insufficient therapeutic efficacy against refractory blood cancers. We are challenging to overcome these disadvantages of current CAR-NK cell therapy and would develop improved CAR-NK cells with safety and sufficient efficacy through genetic modifications and drug treatment, which can survive in the body for a long time, expand when they recognize cancer cells, and efficiently eliminate cancer cells (Figure 4). Furthermore, we aim to build a technical platform for the development of next-generation NK cell therapy, such as optimized CAR for NK cells, developing CAR-NK cells that can be used to treat various cancer types, and developing materials and methods for efficiently expansion and storage conditions for CAR-NK cells while maintaining their high killing activity against cancer cells (Figure 4).
Our goal
To date, there are neither therapeutic drugs that artificially enhance cancer immunity of NK cells nor cancer immunotherapy that can cure cancer by promoting NK cell immunity. In addition, the mechanisms by which NK cells recognize cancer cells through various NK receptors, integrate activation signals in NK cells, and regulate the strength of their killing activity against cancer cells are still not fully understood. By gaining a deeper understanding of NK cells, we aim to develop drugs that enhance NK cell-mediated cancer immunity and next-generation NK cell-based cancer immunotherapies.
For experts
Vision, big picture, and goal
- Our vision is to eradicate cancer.
- Our big picture is to understand NK cells themselves and to elucidate the nature of NK cell-mediated cancer immunity.
- Our goal is to contribute to society by developing effective cancer immunotherapy on the basis of NK cell biology.
To achieve this goal, we dedicate ourselves to research on immunology and cell biology, and work closely with the fields of basic research, translational research, and clinical research to incorporate a wide range of knowledge and technologies. We will conduct our own unique research that incorporates biology, medicine, and engineering, with the aim of treating cancer.
Research background
What are NK cells?
NK cells are lymphocytes that consist of only 2-10% of peripheral blood mononuclear cells. However, NK cells play an essential role in controlling cancer. NK cells are also essential for controlling viral infections of the herpesvirus genus, e.g., cytomegalovirus (CMV), and are also involved in controlling sometimes fatal viral infections, such as hepatitis viruses, Ebola virus, HIV, and SARS-CoV-2. NK cells recognize unhealthy and abnormal cells including cancer cells, then are activated and release cytotoxic granules containing perforin and granzymes toward these unhealthy cells (called target cells), thereby showing the cytotoxicity. At the same time, activated NK cells produce cytokines and chemokines, including interferon-γ (IFN-γ). Their cytotoxic activity and cytokine production are called as effector functions.
Cancer immunity
NK cells and CD8+ T cells are immune cells responsible for cancer immunity. CD8+ T cells recognize cancer antigen-derived peptides presented on major histocompatibility complex (MHC) class I molecules expressed on the surface of cancer cells with a clonal T cell receptor. CD8+ T cells are then activated and exert cytotoxic activity against cancer cells (Figure 1). However, as cancer cells become malignant, they downregulate or lose the expression of MHC class I molecules, thereby evading the cancer immune responses of CD8+ T cells. In general, low or negative expression of MHC class I on cancer cells is observed in 25-75% of cancer patients. On the other hand, NK cells can recognize cancer cells with these low or negative expression of MHC class I, which cannot be eliminated by CD8+ T cells, using a variety of NK receptors, and then are activated and exert the cytotoxicity (Figure 1). Thus, NK cells and T cells have complementary roles in cancer immunity, and both play essential roles in controlling cancer.
Challenges in cancer immunotherapy
Cancer immunotherapy, e.g., immune checkpoint inhibitors as the representative, has established itself as an option for cancer treatment. However, the therapeutic efficacy is still limited and only a limited number of cancer types can lead to complete regression. In addition, current cancer immunotherapies often cause severe, sometimes fatal, side effects. Therefore, given overcoming the treatment resistance of cancer to conventional therapies and achieving synergistic therapeutic effects when used in combination with conventional treatments, there is an urgent need for the development of safe and effective cancer immunotherapies with a different mechanism of action. To date, research and development of cancer immunotherapy is mainly focused on T cells. The major mechanism of resistance to cancer immunotherapy is the downregulation of MHC class I on cancer cells. NK cells can recognize and eliminate these cancer cells that cannot be eliminated by T cells. Since both NK cells and T cells are essential in cancer immunity, in case that the cancer immune responses of NK cells can be implemented, it would be possible to combat cancers that cannot be cured by conventional T cell-mediated cancer immunotherapy. Nowadays NK cells are expected to become a game changer in cancer treatment in the future, however, to date, neither treatments nor drugs to promote the anti-cancer immunity of NK cells have been developed.
Research summary
In the Division of Immune Response, we are mainly investigating cancer immune responses of NK cells. NK cells would be useful for the development of next-generation cancer immunotherapy, because they can recognize, be activated, and eliminate cancer cells that T cells cannot eliminate. However, neither therapeutic drugs to enhance NK cell-mediated cancer immune responses nor NK cell-based cancer immunotherapy that can cure refractory and relapsed malignancies have been developed. For the establishment of a strategy to promote the anti-cancer activity of NK cells, we need a deeper understanding of NK cell biology. First, it is essential to elucidate a series of molecular mechanisms by which NK cells recognize cancer cells using NK receptors, integrate activation signaling through NK receptors, regulate the activation, and exert the cytotoxicity. Furthermore, it is necessary to identify and analyze the functions of novel inhibitory molecules and immune checkpoint molecules that serve as therapeutic targets to enhance the anti-cancer activity of NK cells. We aim to gain the results of these basic research to translational research, thereby leading to the development of drugs that enhance NK cell-mediated cancer immunity and building a proof-of-concept for next-generation NK cell-based cancer immunotherapy. Ultimately, we aim to offer our newly developed NK cell therapies for cancer as a treatment option after verifying its safety and efficacy in clinical trials.
Our research
At present, there is no established therapeutic strategy to activate cancer immune responses of NK cells. NK cells and T cells play essential roles in cancer immunity. Thus, therapeutic modality that enhance NK cell-mediated cancer immunity would be innovative, because the modality possibly achieves synergistic therapeutic effects by combining with conventional cancer immunotherapies to promote T cell-mediated cancer immunity, such as immune checkpoint inhibitors. To enhance NK cell-mediated cancer immune responses, we are taking a cross-disciplinary approach based on immunology, cell biology, biochemistry, bioinformatics, and so on from basic research on immune cell biology of NK cells to translational research and drug discovery.
1. Identification and analysis of NK receptors, their ligands, and immune checkpoints, and drug discovery targeting them
NK cells express a variety of activating NK receptors and inhibitory NK receptors on their cell surface (Figure 2). Activating NK receptors recognize molecules as their ligands that are upregulated on the surface of abnormal cells including cancer cells. The interaction of activating NK receptors with activating ligands transmits activation signals, induces the activation, and regulate the effector function. On the other hand, the majority of inhibitory NK receptors recognizes MHC class I molecules as their ligands and transmit inhibitory signals to suppress activating NK receptor signaling (Figure 2). Normal cells express MHC class I molecules, while many cancer cells downregulate or lose the expression of MHC class I to escape T cell immunity. When NK cells encounter normal cells, NK cells are not activated because they receive inhibitory signals through inhibitory NK receptors in the context of the interaction with MHC class I. Thus, NK cells do not show cytotoxic activity against normal cells (activating NK receptor signals < inhibitory NK receptor signals) (Figure 2). In contrast to normal cells, cancer cells downregulate the expression of MHC class I, resulting in less or no inhibitory NK receptor signaling in NK cells. Furthermore, because cancer cells highly express activating NK receptor ligands, NK cells are activated by activating NK receptor signaling and exert the cytotoxic activity against cancer cells (activating NK receptor signals > inhibitory NK receptor signals) (Figure 2). Even if the expression of MHC class I is maintained on cancer cells, damaged cells, transformed cells, stressed cells etc., in case the expression of activating NK receptor ligands is significantly increased, NK cells are activated by activating NK receptor signals and show the cytotoxicity (activating NK receptor signals > inhibitory NK receptor signals) (Figure 2). Thus, the magnitude of activation and effector function of NK cells is quantitatively determined by the balance and sum of the signals through activating NK receptors and inhibitory NK receptors. If the sum of the activating NK receptor signals exceeds the sum of the inhibitory NK receptor signals, NK cells are activated and show effector functions (Figure 2). Resultingly, NK cells can selectively exert the cytotoxicity against cancer cells and eliminate them (Figure 2). Therefore, the identification and functional analysis of NK receptors, their ligands, and downstream signaling molecules of these NK receptors are the most important topics in NK cell biology.
However, all NK receptor-ligand interactions have not yet been determined. Also, ligands for NK receptors known to be involved in cancer immune responses are often unidentified. The identification of activating and inhibitory NK receptors that recognize molecules as ligands on cancer cells is of great significance, because it can be directly linked to artificial regulation of NK cell-mediated cancer immunity. Moreover, when NK cells recognize cancer cells, they simultaneously receive signals through multiple activating and inhibitory NK receptors. However, it is not fully understood how these activating and inhibitory NK receptor signaling is integrated and regulated in NK cells. We try to identify and functionally analyze these unknown NK receptors and their ligands, as well as molecules involved in the integration of NK receptor signaling. We will determine their roles in cancer immunity and then develop therapeutic approaches to promote NK cell activity, leading to clinical applications.
In addition, there are limited studies on molecules that suppress cancer immune responses of NK cells. One possible strategy for enhancing NK cell-mediated cancer immunity is to develop inhibitors of critical regulators, e.g., immune checkpoint molecules or inhibitory factors expressed by NK cells, or to target genes encoding these regulators by genetic modification. However, since NK cells do not highly express known immune checkpoint molecules, it is necessary to identify and analyze the functions of new inhibitory factors and immune checkpoint molecules that can serve as therapeutic targets. Signaling molecules that inhibit a variety of activating NK receptor signaling pathways would be promising a promising target for drug discovery to enhance the anti-cancer activity of NK cells. However, to date, such inhibitory molecules have not been identified. Recently, we have been focusing on such inhibitory factors and analyzing the functions of NK cell immune checkpoint molecules which we have newly discovered. Currently, we are investigating the detailed molecular mechanisms by which the immune checkpoint molecules suppress NK cell-mediated cancer immunity. Also, we try to discover approved drugs and synthetic chemical compounds that inhibits the function of these immune checkpoint molecules; that is, drugs to promote the anti-cancer activity of NK cells (NK cell activator), by several approaches including immunology, cell biology, biochemistry, structural biology, artificial intelligence, and chemical library screening.
2. Elucidation of the molecular mechanisms underlying the differentiation and function of memory NK cells
One of the most notable features of the immune system is immunological memory. Immunological memory is a phenomenon in which, as typified by vaccines, a faster and stronger immune response is mounted when re-exposed to foreign substances and pathogens that have previously exposed, and the improved host defense against them are maintained for months, years, sometimes even a lifetime. The immunological memory of CD8+ T cells is also necessary for a sustained and effective cancer immune response. It has been believed that only T cells and B cells that can differentiate into memory cells are responsible for immunological memory. By contrast, since their discovery, NK cells have been thought to be short-lived cells that turn over within 1-2 weeks in vivo and die after showing effector functions against target cells. Therefore, it was believed that NK cells have no ability of the antigen-specific proliferation or the differentiation into long-lived memory cells like CD8+ T cells, thereby not involving in immunological memory. However, in recent years, a discovery has been made that overturns the conventional theory: after cytomegalovirus (CMV) infection, mouse NK cells expressing the activating NK receptor Ly49H and human NK cells expressing the activating NK receptor NKG2C recognize CMV-infected cells, undergo antigen-specific proliferation, and differentiate into memory NK cells that contribute to long-term protection against the infection (Figure 3). These activating NK receptors specifically recognize CMV proteins and their fragments expressed on CMV-infected cells. Both activating NK receptors associate with the signaling adaptor molecule DAP12 and transmit activation signals via downstream signaling molecules Syk and Zap70. Activated NK cells show the cytotoxicity to eliminate CMV-infected cells. Thus, these CMV-specific NK cells play an important role in controlling CMV infection at the early course of infection. These activated NK cells then undergo clonal expansion and persist in the body as long-lived NK cells. These long-lived NK cells were defined as “memory” NK cells, because (1) they are long-lived NK cells that have undergone clonal expansion; (2) they display unique cell surface antigens, transcriptomic profile, and epigenetic landscape that are distinct from those of naïve and activated NK cells; (3) they show superior effector functions in response to activating NK receptor signaling in vitro to naïve NK cells; (4) they have the ability of secondary expansion against secondary CMV infection; and (5) they confer improved host protection against CMV challenge, as compared with naïve NK cells (Figure 3). Intriguingly, memory NK cells show enhanced effector functions in response to activation signaling through all activating NK receptors expressed on these cells. This would be possibly because the memory differentiation lowers threshold for activating NK receptor signaling through comprehensive changes in transcriptomic and epigenetic landscapes, enabling memory NK cells to exert augmented effector functions “in all directions” regardless of the types of activating NK receptors. In fact, memory NK cells show augmented anti-cancer activity after stimulation of activating NK receptors DNAM-1 and NKG2D, both of which are important for anti-cancer immunity of NK cells, and also show strong antibody-dependent cellular cytotoxicity (ADCC) via the activating NK receptor CD16. Memory NK cells can proliferate in response to recurrent CMV infection following bone marrow or organ transplantation, contributing to control of CMV infection and CMV-associated adverse events. Furthermore, memory NK cells may prevent the aggravation of HIV and SARS-CoV-2 infections. As an important clinical significance of NK cell memory, it has been reported that memory NK cells reduce the relapse rate and improve the survival rate of cancer patients with hematological malignancies by eliminating minimal residual disease. Furthermore, the strong ADCC activity of memory NK cells may contribute to the therapeutic effect of antibody drugs, such as Trastuzumab, Cetuximab, Rituximab, Avelumab, whose major mechanism of action is ADCC.
While we are engaged in research into NK cell memory with a view to its application to cancer immunology, we have discovered that memory NK cells have augmented anti-cancer activity. In addition to the strong anti-cancer activity, memory NK cells have attractive functional features for cancer immunity: long-term survival, enhanced effector functions, and proliferative ability. Therefore, the elucidation of the regulatory mechanisms underlying the differentiation and function of memory NK cells is of importance for the development of cancer immunotherapy utilizing NK cell memory. Through the literature, the roles of various NK cell-related molecules, such as NK cell receptors, cytokines, signaling molecules, and transcription factors, were determined. However, it is hard to say that the nature of the immunological memory of NK cells has been unveiled. Moreover, since memory NK cell differentiation has not been observed in cancer patients to date, it is presumably because environmental factors and signaling, which are necessary conditions for the optimal formation of NK cell memory, should be lacking in cancer. In the future, it will be necessary to deepen our understanding of NK cell memory by (1) searching for diseases in which memory NK cell differentiation is confirmed, (2) studying signaling pathways such as NK receptor-ligand interactions and cytokines that trigger memory NK cell differentiation, and (3) identifying and analyzing the functions of transcription factors and epigenetic factors that form the molecular identity of memory NK cells. We are conducting basic research to elucidate the molecular mechanisms underlying NK cell memory. At the same time, by elucidating the regulatory mechanisms, we aim to apply NK cell memory formation or memory NK cells themselves to cancer immunity, e.g., enhancement of anti-cancer activity of NK cells and reactivation of tumor-infiltrating exhausted NK cells by artificially promoting memory NK cell differentiation using genetic modification, genome editing, and treatment of chemical compounds.
3. Establishment of next-generation NK cell-based cancer immunotherapy
The efficacy of cancer immunotherapy is still insufficient and only a limited number of cancer types can lead to complete regression. One of the new therapies is cancer-specific chimeric receptor (Chimeric Antigen Receptor, as known as CAR) T cell therapy. T cells engineered to express CAR, an artificial activating receptor specific for cancer antigens, specifically recognize cancer cells, are activated by CAR activation signaling, and show cytotoxic activity against cancer cells. CAR T cells are manufactured by which peripheral blood T cells from cancer patients are isolated and CAR-transfected T cells are infused back into these patients. CAR T cell therapy shows high therapeutic efficacy against refractory and relapsed B cell malignancies. However, serious or fatal adverse effects, such as cytokine release syndrome and neurotoxicity, frequently occur. In addition, since CAR T cells need to be manufactured individually from the patient’s own peripheral blood T cells, there is a risk that treatment opportunities may be missed in case of patients with rapidly progressing cancer types, as well as the manufacturing CAR T cells may fail in a certain percentage. NK cells are an attractive tool to overcome these challenges of CAR T cells. Recently, CAR-NK cell therapy has been developed as a new modality for activating NK cell-mediated cancer immunity. A recent clinical trial using CAR-NK cells which were prepared using allogeneic umbilical cord blood NK cells has reported the effectiveness of CAR-NK cell therapy against B cell malignancies. In this clinical trial, no serious adverse effects including grade 2 or higher graft-versus-host disease, cytokine release syndrome, or neurotoxicity, were confirmed, indicating that CAR-NK cell therapy is safer than CAR T cell therapy. In addition, CAR-NK cells can be manufactured from allogeneic umbilical cord blood or peripheral blood from healthy individuals. Moreover, CAR-NK cells can be expanded and stored, so they are likely to be used as a cost-effective “off-the-shelf” product in the future. Since CAR-NK cells can recognize cancer cells not only by the CAR but also by endogenous activating NK receptors, these activating NK receptor signals also may enhance the cytotoxicity against cancer cells. However, current CAR-NK cells are short-lived and have insufficient proliferative activity in cancer patients, resulting in low therapeutic effects against refractory and relapsed B cell malignancies. In addition, the CAR structure used for engineering CAR-NK cells is a second-generation CAR construct (c.f. the latest CAR construct for CAR T cell therapy is fifth-generation), although the antigen receptors, costimulatory molecules, signaling molecules, and critical cytokines for functions of NK cells and T cells are not necessarily identical. Thus, the structure of CAR has to be optimized for NK cells. To overcome these challenges of the current CAR-NK cell therapy, we aim to establish a method for manufacturing improved CAR-NK cells with enhanced anti-cancer activity, long-term survival ability, and proliferative activity through gene transfer, genome editing, optimization of cytokine culture, chemical treatment, etc. (Figure 4). It is also known that tumor-infiltrating NK cells are dysfunctional (exhausted) in tumor microenvironment. We are also attempting to engineer CAR-NK cells that are less prone to exhaustion through genetic modification. At the same time, by elucidating the molecular mechanisms of enhanced therapeutic effect of CAR-NK cells, it might be possible to identify new therapeutic target in NK cells, which can be applied to the research and development of improved NK cell-based cancer immunotherapy with even higher therapeutic effects. In addition, we aim to establish various fundamental technologies, such as methods and materials for efficient proliferation of NK cells (combinations of feeder cells and cytokines), methods for efficient gene transfer and genome editing for NK cells, optimized CAR constructures specialized for NK cells, development of CAR that can be used for a wide range of cancer types, establishment of culture conditions that enable expansion of CAR-NK cells while maintaining high anti-cancer activity, optimal storage condition for banking, and development of a program to select CAR-NK cell batches that are safe and maximize therapeutic effects based on the HLA, expression patterns of NK receptors, and CMV infection profiles of cancer patients and donor NK cells (Figure 4). Through these research and development efforts, we will build an R&D platform for next-generation NK cell therapy.
Members
[Keywords and skills]
NK cells, NK receptors, immunological memory, type 1 innate lymphoid cells
Immunology, Cell biology, Hematology, Molecular biology,
Cancer Immunology, Cancer Immunotherapy, Viral infection
Flow cytometry, primary cell culture, bone marrow transplantation
researchmap
Google Scholar
2003-2005: Kobe University (2005 M.S.)
2013-2016: Mie University Graduate School of Medicine (2016 Ph.D.)
2006-2013: Researcher, Immunofrontier, Inc.
2013-2015: Assistant Professor, Mie University Graduate School of Medicine
2016-2018: Research Assistant Professor, Graduate School of Pharmaceutical Sciences, University of Shizuoka
2018-2021: Associate Professor, Nagasaki University Graduate School of Biomedical Sciences
2022-present: Unit Leader, Division of Translational Oncoimmunology, Aichi Cancer Center Research Institute
2) Development of novel therapeutic strategies for cancers resistant to immunotherapy.
2) Improving TCR affinity specific for neoantigens
JSPS Research Fellow in 2021, Special Assistant Professor in International Advanced Research at Chiba University in 2022. Current position since April 2025.
Recruiting
In Division of Immune Response, we are conducting basic research to elucidate the molecular mechanisms underlying cancer immunity of NK cells, and also translational research aiming at the clinical application of NK cell-mediated cancer immunity. We welcome enthusiastic researchers with diverse research backgrounds, including immunology, cell biology, molecular biology, biochemistry, cell engineering, bioinformatics, cancer therapeutics, cancer biology, and medicine. We have a variety of positions available, including Researcher (tenured and tenure-track), JST Research Assistant (funded RA for PhD student, supported by JST FOREST), Research Resident, Affiliated Graduate Students, Research Assistant (part-time), and Voluntary Trainee. If you are interested in our research, please feel free to contact me by Email 24/7.

