Department of Pathology and Molecular Diagnostics
Introduction and Welcome
The Department of Pathology and Molecular Diagnostics provides three major diagnostic services: histopathology, cytopathology, and molecular pathology. Since the establishment of the Aichi Cancer Center in 1964, our department has been one of the leading laboratories in Japan, offering broad expertise in diagnostic pathology and molecular diagnostics of cancer, and performing creative research in a variety of cancer types including lymphoma, cancers of the lung, pancreas, GI tract, and head and neck. Our department is an ISO15189-accredited laboratory and currently handles 9,000 cases of histological diagnoses and 6,000 cases of cytopathological diagnoses a year.
The Molecular Pathology lab performs DNA and RNA based assays relevant to the choice of molecular targeted drugs that are most effective according to the genetic alterations that each tumor has. The lab also works in collaboration with the Center for Cancer Genomic Medicine and offers “Cancer Genomic Medicine” by performing next-generation sequencing-based tests, including FoundationOneCDx, OncoGuideTM NCC Oncopanel, MSK-IMPACT, and Whole Exome Sequencing.
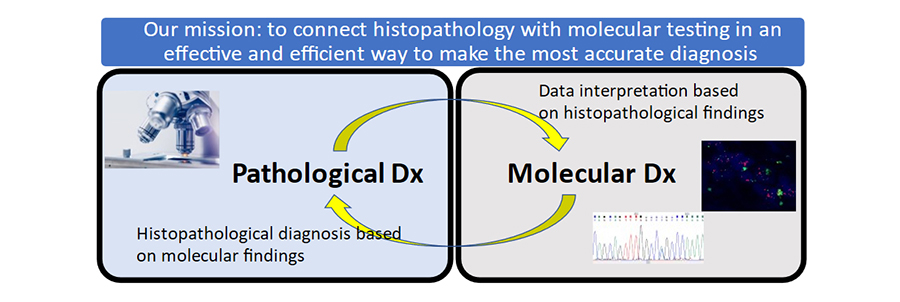
The amount of knowledge of molecular features of cancers is now overwhelmingly large. Our mission is to connect histopathological assessment with molecular features in the most effective and efficient way. Using tissue from each patient, we do our best to find as much information as possible to make an accurate diagnosis, which ensures the correct and effective treatment for the patient.

Waki Hosoda, M.D., Ph.D.
Chief
Staff
Waki Hosoda, M.D., Ph.D. (Chief)
EXPERTISE
Histopathology, cytopathology, molecular diagnosis of cancer
RESEARCH INTERESTS
Pancreatic tumors, neuroendocrine tumors, molecular diagnostics of cancer
Eiichi Sasaki, M.D., Ph.D.
EXPERTISE
Histopathology, cytopathology
RESEARCH INTERESTS
Head and neck tumors, soft tissue and bone tumors
Haruna Yagi, M.D.
EXPERTISE
Histopathology, cytopathology
RESEARCH INTERESTS
Salivary gland tumors
Katsuhiro Masago, M.D., Ph.D.
EXPERTISE
Molecular diagnosis
RESEARCH INTERESTS
Molecular oncology, lung cancer
Divisions
Histopathology
The laboratory provides expert evaluation of histopathology using:
- Light microscopy
- Special stains
- Immunohistochemistry (250 antibodies)
- In-situ hybridization (30 probes for biomarkers, sarcomas, lymphomas, etc.)
Our service also includes:
- Surgical pathology
- Second opinions (patients can consult with or obtain a second opinion on pathological diagnosis from our staff via physicians)
- Autopsy (performed if it is helpful in providing comfort for families seeking to establish cause of death or to resolve questions about treatment)
Cytopathology
Cases are screened by 5 staff cytotechnologists with expertise in cancer cytopathology and signed out by 4 board-certified pathologists.
Major areas of requests include:
- Gynecological cancer
- Breast cancer
- Thoracic cancer
- EUS-FNA (including rapid on-site evaluation)
- Pancreatobiliary cancer
- Bladder cancer
Molecular Pathology
Services provided
- EGFR, KRAS, BRAF, HER2 mutations for lung cancer
- ALK, ROS1, RET, NTRK1/2/3 fusions for lung cancer
- MET exon 14 skipping for lung cancer
- Targeted 46-gene panel for lung and thyroid cancers
- KRAS, NRAS, BRAF mutations for colorectal cancer
- HER2 amplification for breast, gastric, colorectal, and salivary gland cancers
- KRAS, CTNNB1 mutations for pancreatic tumors
- ER, PgR, Ki-67 tests for breast cancer
- PD-L1 immunostaining for the use of immune checkpoint inhibitors
- MSI test
- MMR protein immunostaining
- Fusion gene detection for sarcomas (FISH, RT-PCR; EWSR1, SYT, CHOP, etc.)
- MDM2 amplification for sarcomas
- CTNNB1 mutation for desmoid tumor
- Mutation analysis of IDH1/2, H3.3A/B for bone tumors
- Fusion gene detection for lymphomas (FISH; MYC, BCL2, API2-MALT1, etc.)
- IGH, TCRG Clonality assay for lymphomas
- FoundationOne CDx #
- OncoGuideTM NCC Oncopanel System #
- myChoice CDx for ovarian cancer
- Oncotype DX for breast cancer
# Performed in collaboration with the Center for Cancer Genomic Medicine

